| Revision as of 02:39, 29 December 2006 editLight current (talk | contribs)30,368 edits rv unauthorised dletion← Previous edit | Revision as of 02:40, 29 December 2006 edit undoHipocrite (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers22,615 edits rvNext edit → | ||
| Line 280: | Line 280: | ||
| I was reading about the Theory of Special Relativity in ], and ] said that if you chase a beam of light at light speed, the beam of light will continue to get farther away from you at the speed of light. This made my head hurt. Is it just one of those strange quirks of physics that you just have accept because there is proof for it, no matter how weird it is; or is there a real ''qualitative'' reason behind this phenomenon? Interesting stuff, this special and general relativity. :-) ]<sup>]</sup> 01:33, 29 December 2006 (UTC) | I was reading about the Theory of Special Relativity in ], and ] said that if you chase a beam of light at light speed, the beam of light will continue to get farther away from you at the speed of light. This made my head hurt. Is it just one of those strange quirks of physics that you just have accept because there is proof for it, no matter how weird it is; or is there a real ''qualitative'' reason behind this phenomenon? Interesting stuff, this special and general relativity. :-) ]<sup>]</sup> 01:33, 29 December 2006 (UTC) | ||
| :The fact that the ] is exactly the same in ''all'' ]s is an ] (that is, an assumption) of the ]. It does not seem to have been intuitively obvious to anyone except Einstein himself, but it does turn out to result in theories that are correct to very high precision. -- ] 01:55, 29 December 2006 (UTC) | :The fact that the ] is exactly the same in ''all'' ]s is an ] (that is, an assumption) of the ]. It does not seem to have been intuitively obvious to anyone except Einstein himself, but it does turn out to result in theories that are correct to very high precision. -- ] 01:55, 29 December 2006 (UTC) | ||
| == Is it possible to turn woman into a sex addict? == | |||
| If I inject some of form parasite into an unwilling women say ], would she turn into a sex addict. If I'm caught would I go to prison? ] 01:29, 27 December 2006 (UTC) | |||
| :With regard to your second question, I would hope so. --] ] 01:35, 27 December 2006 (UTC) | |||
| :*Extending my response out of order (if I'm allowed): I had no idea that there would be such controversy about this trollish subject matter and had hoped (naively) that saying such behavior would result in prison time (if caught) would end the thread. It seems that that is not the case at all and that the un-crisp boundaries of proper vs criminal behavior are now being debated under this heading and that is wrong in this venue. If I had any hand in this by remarking that it was "my hope" that such behavior (implicitly) would be criminal then I'm sorry for inflaming this sorry subject and it's resultant debate. If on the other hand something useful happens well, that's WP.--] ] 03:43, 27 December 2006 (UTC) | |||
| :Well you have linked the appropriate page. Have you read it? Esp this bit: | |||
| <blockquote> | |||
| ''For women: | |||
| * A tendency to be more outgoing, friendly and more promiscuous | |||
| * They are considered more attractive to men compared with non-infected controls | |||
| "In short, it can make men behave like alley cats and women behave like sex kittens" — Nicky Boulter | |||
| '' | |||
| </blockquote> | |||
| --] 01:37, 27 December 2006 (UTC) | |||
Revision as of 02:40, 29 December 2006
| Reference Desk |
| |||||||||
How to ask a question
| |||||||||
|
| ||||||||
|
After reading the above, you may ask a new question by clicking here. Your question will be added at the bottom of the page. | |||||||||
How to answer a question
|
| ||||||||
December 26
Orbit of a particle
Does the orbit of a particle (whose radius is of course greater than zero) turn into a spin when the radius of the particle's orbit is reduced to zero? 71.100.6.152 01:10, 26 December 2006 (UTC)
- What are we talking about here, classical mechanics or quantum mechanics? —Keenan Pepper 01:49, 26 December 2006 (UTC)
- How about an answer for both. 71.100.6.152 03:20, 26 December 2006 (UTC)
- Quantum mechanical spin is an internal degree of freedom of a particle which is unrelated to motion in space. It is not related to orbital motion, and moreover the total spin of a particle cannot change (total angular momentum can be affected by external forces). Part of the strangeness of QM spin is that one cannot think of a QM particle as a body with finite radius. Classical spin is also somewhat different from spatial angular momentum. A particle which is in an orbit with some angular momentum does not necessarily spin. If every point in a solid body is at rest with respect to all other points in the body, it is not spinning. --Bmk 16:11, 26 December 2006 (UTC)
- Assuming for the moment that the core or the Earth and Moon are fix relative to their surfaces they still spin. The spin of the Earth is not a core to surface relation but rather an Earth to Sun, Moon and Stars relation while its orbit is a relationship to the Sun. The Moon orbits the Earth yet does not appear to spin by observers on the Earth but in relation to the Sun and stars it does spin in sync with its orbit around the Earth so your classical physics answer puzzles me. It seems like what you are saying is that if the surface of a body moves in relation to its core then it spins and that this is not the same as its surface orbiting its core. Maybe the problem is just semantics. 71.100.6.152 21:16, 27 December 2006 (UTC)
- Mmm - i see why that would be puzzling. However, there is something measurably different about a body that is spinning and a body which is not spinning; it actually is not a semantic difference, but quite quantifiable. You can get a feel for the difference if you consider a wet basketball; if you spin it, the water will be thrown off the ball - this is clearly not a matter of perspective, but something intrinsic to the system. Consider reading about inertial reference frames, and angular momentum. Hope this helps --Bmk 05:34, 28 December 2006 (UTC)
- May then I conclude that anything having Centripetal force is spinning and that this is not a characteristic force of an orbit? (...i.e, that the Moon is not moving away from the Earth because of centripetal force.) 71.100.6.152 18:01, 28 December 2006 (UTC)
How to seep and steep herbal teas and remedies
I have encountered the terms “SEEP” and “STEEP” (I may have misspelled the second term) in relation to the creation of herbal teas and remedies. I am a bit confident that “seep” is the process used in my coffee maker. These terms are often used in hiking and camping books in the editable plants section. These books never seem to explain the method. I have been unable to find a source explaining the processes (both in modern terms and ancient methods). Also the terms always seem to be two very different processes.
- To "steep" is to leave an object (such as a tea bag) in (usually hot) water to disperse flavor, etc. Your coffeemaker is doing an action similar to steeping. To seep is for water to permeate an object (usually used in a way that the "seep" is unfavorable, like "wow, the rain seeped through my tent"). Seep is usually a general term, and steep is a term used in making drinks. I'm guessing your recipes are telling you to steep tea bags or loose tea - this is just a term for sticking it in hot water for a few minutes. --Wooty Woot? contribs 02:23, 26 December 2006 (UTC)
- Agreed. Also, since many people are unfamiliar with the above usage of "steep", they may mistakenly substitute the word "seep", since that word, they know, does have something to do with liquids. StuRat 13:55, 27 December 2006 (UTC)
Budgie video on YouTube
Just found this: http://www.youtube.com/watch?v=Xe2Jb3U21vA
There's some logic at play here but I'll be damned if I know what it is. Any suggestions? --Kurt Shaped Box 02:06, 26 December 2006 (UTC)
- Bored housepets enjoy play too? Vranak
- Yeah, I know it looks like he's just messing around but the bird's glances back and forth between the string of beads and the lone bead make me think that he's actually trying to do something with them position-wise. Sometimes he'll walk halfway to one or the other, stop to think for a moment, then turn back. --Kurt Shaped Box 23:46, 26 December 2006 (UTC)
- Fairly simple. Budgies, being parrots, are quite intelligent as birds go, and like to play. They like to play with novel things especially, and it is quite natural that the temptation of the larger object grows while playing with the similar, smaller object. Thus, the budgie is enticed to give the string of beads a try. But the accessible string is too short to handle comfortably with such a small bill, and it is apparent that they cannot be thrown around with so much vigor. They look as if they might be a better fun object, but actually are lamer. The budgie finds it increasingly hard to decide whether to give the string of beads a try or not, finally does, and on finding them lame walks away. It is like the decision a four-year-old has when having to choose between a large, light box and a small, heavy one. The one bead is known to be fun to the budgie, but eventually, it becomes boring enough to giuve the other stuff (which lokks enticing and not at the same time) a try. Dysmorodrepanis 04:52, 28 December 2006 (UTC)
Capacitance of batteries.- its a hard one this!
Any one got any idea of the inherent ac capacitance between the terminals in batteries and whether this capacitance bridges the internal resistance?? In other words, what determines the battery's impedance? 8-)--Light current 02:27, 26 December 2006 (UTC)
- Well, looking at the battery purely as a black box, there must be capacitance both between the two terminals of the battery and also between the battery and the surrounding environment. I'd guess that the capacitance to the environment is pretty small. I'd further guess, given the construction of most batteries, that the terminal-to-terminal capacitance is pretty small also, although some NiCd and lead-acid gelled-electrolyte batteries use a rolled-up construction that probably has quite a bit of inherent capacitance.
- If you're really interested and you have a capacitance meter, it'd be pretty easy to measure: just put a known-value blocking capacitor in series with the battery, short the combined battery-capacitor series circuit (so the blocking cap charges up and no inrush current damages your meter), attach the series circuit to your meter, and unshort the series circuit. Take the reading you get from the meter and, using the known value of the blocking capacitor, solve the series circuit for the unknown capacitance of the battery. If the blocking cap is of large value compared to the reading of the meter, then just take the reading of the meter directly as the capacitance value of the battery.
- Needless to say, I have never measured this nor heard of anyone doing so and of course normally we decouple the batteries from the circuit with a large capacitor. So one question is, assuming a baatery with low internal reistance, like a lead acid job, is it really neccessary to put large electrolytics across it for low freq decoupling. I am ignoring the inductance of the battery leads as this will only be important at the higher frequencies.
- And yes of course Atlant, the method you suggest should work well at the spot frequency of the meter. I suspect, though, that the 'capacitance' actually rises with decreasing frequency (due to the chemical reaction effects) such that, at DC, you actually end up with the 'capacity' of the battery Q =it =CV. So C = it/V which is going to be quite large even for a 1 A hr cell. --Light current 18:23, 26 December 2006 (UTC)
- You are correct, of course, that at low frequencies classical capacitance becomes meaningless for a battery; thanks for pointing that out. Also, apropos a topic you and I were discussing quite some time ago, folks who build real neighborhood-thumping car stereos often put large "ultracapacitors" in parallel with their car batteries (or at least, near the welding cables with which they feed their power amplifiers). They do this to provide the maximum transient power to their window-breaking amplifiers. And the manufacturers of hybrid cars are also looking at this, seeing as how ultracapacitors can be very rapidly (and efficiently) charged and discharged without the cycle-life issues that affect classical batteries. So they're investigating a two-level energy storage hierarchy with ultracaps first and batteries second.
- Actually, I think the capacitance of batteries is fairly huge, up in the Farads realm, since it arises from the atomically-thin insulating "Double layer" on the surface of the battery plates. The electrolyte is a conductor, and so are the battery plates, so the only insulator of significance is the surface double-layer where the charge-pumping effect originates. If we construct a battery where the two plates are made of the same metal, then we end up cancelling out any charge-pumping process, and this produces zero output voltage. Yet in that case the capacitance of the double-layer still exists, and as long as we avoid driving chemical reactions by applying voltages above ~1v to the plates, we can measure the battery's capacitance directly. It's the capacitance of two double-layers in series, and can be as high as tens of Farads. Don't forget that supercapacitors and ultracapacitors are based on just this same effect. They use carbon-powder electrodes immersed in sulfuric acid solution, and both of these materials are conductors. The dielectric in the ultracapacitor is the double layer, but in order to be useful at voltages > 1V, these capacitors use many double-layer capacitors wired in series. --Wjbeaty 05:55, 27 December 2006 (UTC)
- If I get some time, I'll wander off to our precision LCR bridge. Alternatively, a capacitance of Farads ought to show up as a huge pulse of current if the battery is short circuited; that ought to be pretty easy to detect with a 'scope, current probe, and low-value series resistor (so I don't magnetize the current probe too badly; ours is rated 30A peak).
- Okay, I did some research. I used the test rig I recommended above (with a capacitor in series with the battery) and our HP 4284A Precision LCR bridge. My series capacitor was a "1000" μF aluminum electrolytic which was actually running on the wimpy side at about 900 μF. I took readings at 20 Hz, 100Hz, 10KHz, and 10 KHz, stopping there because my rig was apparently starting to resonate and had gone noticeably inductive at 100 KHz. For batteries, I used a brand new alkaline "AA" (LR6) cell and a stone cold dead alkaline AAA cell. The resulting battery capacitances:
- 20Hz: AA=2991 μF AAA=946 μF
- 100Hz: AA=2907 μF AAA=287 μF
- 1KHz: AA=2332 μF AAA=161 μF
- 10KHz: AA=1086 μF AAA=99 μF
- I think it's safe to say that ordinary alkaline cells have "hundreds to thousands of microFarads" of capacitance, but there's no evidence that this design of batteries has "Farads". And I also think it's safe to say that Light current's hypothesis is also born out: the dead AAA showed a very noticeable rise in "capacitance" as the frequency dropped, quite possibly due to electrochemical storage rather than real capacitance. It's interesting that the fresh ("fully-charged") battery didn't seem show much of this effect. Now, who wants to do the same experiment with a "rolled-construction" battery, where it wouldn't surprise me to see much higher capacitance values, including the "ultracapacitor" effects hypothesized by Wjbeaty?
- Atlant these results are extremely interesting! And thanks so much for taking time to do these tests (especially at work!). I just wondered if your last frequency reading should be 10 kHz.
- Its intersting to note the reduced capacitance of the 'dead' cells cf the new ones. This may be due to the incresed esr of the 'dead' ones. Actually if your bridge does Q factor (or Dissipation) that would indicate the series resistance!
- The results do indeed seem to confirm my gut feeling that healthy batteries do indeed have sufficient capacitance for general low frequency decoupling purposes. (which maybe is how designers of cheapo transistor radios got away with out big storage caps. More thoughts later.8-)--Light current 19:35, 27 December 2006 (UTC)
- "Hz" versus "KHz" -- right you are. Fixed now.
- Also, please note that the dead cell was physically smaller (a "AAA" versus a "AA" live cell), so the comparison must be taken with several grains of salt. I didn't have live and dead cells of the same size at hand.
- I was reading ESR rather than Q, but nothing about those results was startling. The data's at work; I'll post it tomorrow if you like.
- Yeah thats really good of you to do that! OK understand you were measuring esr as well: so your bridge resolves the 2 components? I look forward to the results! --Light current 23:18, 27 December 2006 (UTC)
- Yes, the bridge measures the vector impedance and will then display it to you in your choice of units (so C+Q, C+ESR, L+Q, L+R, etc.) As I write, I'm finishing the discharge of a mostly-dead AA cell; although it's not the same brand as my "new" AA, this ought to give us a better comparison between "new" and "dead" than the new AA -- dead AAA comparison I offered earlier. Later, I'll take and post the data.
Riskiness Rankings
Just about every country has some sort of environment conservation legislation. This legislation often ranks species by how close they are to becoming the next Dodo or Pyrenean Ibex. So there are many systems for ranking plants and animals. Often independent bodies also do ranking (such as the World Conservation Union).
I'm trying to flesh out a list of as many of these ranking systems as possible with:
- What are the categories for the ranking system?
- Who's the group or organisation who assigns the rankings?
- What country or countries are involved?
- What's the Act that makes it official? (if any)
- Does the rank apply to the global population or only the one in that country?
Just about every country has a system, so please check if your country, or your favourite country, to the list on the Conservation status page. If you don't know the answer, try googling. There are a lot of Acts and systems and it's a monumental task putting them all together. Any help appreciated. —Pengo 05:08, 26 December 2006 (UTC)
Some more specific questions:
- Do threatened species of Australia protected under the [[Environment Protection and Biodiversity Conservation Act 1999
EPBC Act]] have to be threatened globally, or just in Australia? (the legislation looks vague: see part 7, but perhaps there are specific cases or something?
- Same question for the US's EPA. Do species need to be threatened globally or locally to qualify? (Canada only considers how threatened a species is within its borders)
- What categories or protection is there in
New Zealand, Argentina, Belgium, South Korea, Cyprus, Seychelles, Mauritius, Libya, Barbados, Tonga, Chile, Uruguay, Puerto Rico, (your country here)...? —Pengo 05:42, 26 December 2006 (UTC)
- I am pretty sure that the EPA is governed in this respect primarily by the Endangered Species Act. In this act, species are considered threatened if they are close to global extinction. Species can be either "threatened" or "endangered", the latter defined as closer to extinction. Though the law's mandate is primarily for the USA there are also some stipulations in it about international cooperation which I have not parsed through. --24.147.86.187 14:39, 26 December 2006 (UTC)
electroscope
A zinc plate is connected to a electroscope. if the electroscope is negatively charged, when ultraviolet radiations falls on the zinc plate, the leaves of electroscope fall down. if the electroscope is positively charged, nothing happens to the leaves. why? —The preceding unsigned comment was added by Skanthckd (talk • contribs) 06:52, December 26, 2006 (UTC).
- What does it mean for something to be negatively charged? Read up on electric charge. And what does ultraviolet radiation (no plural please; "radiation" is a mass noun) do? Read up on the photoelectric effect. Combine the information you find in these two articles. By the way, it was Einstein's explanation of the photoelectric effect won him the Nobel Prize in physics. --Lambiam 09:59, 26 December 2006 (UTC)
- Well, the electroscope is obviously losing negative charge somehow. How do you think this could happen ? How does illumination with ultraviolet light cause this ? How quickly is charge lost ? What happens if you change the brightness of the UV light ? Would light of other wavelengths have the same effect ? The best way to find an answer to your question is to do some experiments with a real electroscope. If this is not possible, you can always read our article on photoelectric effect instead. Gandalf61 10:02, 26 December 2006 (UTC)
- Hey, what about electroscope for additional info ?--Light current 21:32, 26 December 2006 (UTC)
Eyes
Are the eyes of a near-sighted person smaller than the eyes of a regular person? And does it look weirder because when i take my glasses off and i took a picture off me i look sooooooooo weird.
- Farsightedness (hypermetropia) can be caused (one of the reasons) by the eyeball being too small but regarding near sightedness (myopia), nothing has been mentioned. But I suppose that myopia can be caused (one of the reasons) by the eyeball being too long -- WikiCheng | Talk 08:56, 26 December 2006 (UTC)
- Near-sighted people may have a tendency to squint when trying to focus on something in the distance without corrective glasses. This may look strange if you're not used to seeing it, but you would almost certainly be aware of doing this. The glasses make your eyes appear slightly smaller, so taking them off should actually have the converse effect to people (possibly including you) used to seeing your face with glasses on. But aren't you used to seeing yourself without glasses in the mirror? --Lambiam 09:46, 26 December 2006 (UTC)
- I think I saw some stuff on Howstuffworks. Here's some. ] - Nearsightedness. And ] about corrective lenses. And a comparison of refractive vision problems in general - ]. Hope that helps!! FruitMart07 03:04, 27 December 2006 (UTC)
Friction
Please help me solve this problem-
A small block of mass 'm' is kept at the left end of a larger block of mass 'M' and length L .The system can slide on a horizontal road.The system is started towards right with an initial velocity 'v'.The friction coefficient between the road and the bigger block is 'u' and that between the blocks is u/2.Find the time elapsed before the smaller block separates from the bigger block.
Thank you. —The preceding unsigned comment was added by 59.93.67.55 (talk • contribs) 10:03, December 26, 2006 (UTC).
- Is this literally how the question is phrased? Letting o indicate the smaller block and X the larger block, I can imagine (at least) two possible initial situations:
XXXXXXXXXX XXXXXXXXXX ------> _oXXXXXXXXXX__________
- Situation 1: smaller block strictly to the left of the larger block
o XXXXXXXXXX XXXXXXXXXX ------> __XXXXXXXXXX__________
- Situation 2: smaller block at the left on top of the larger block
- In situation 1, it is not clear how the friction between the blocks plays any role. The coefficient of friction for the smaller block is not given, so it would be impossible to tell what will happen. If this is a homework assignment, then I assume the intention is that there is a definite solution, ruling out this interpretation of the question. (Also, homework problem statements tend not to provide irrelevant data, like L and the u/2 would be).
- In situation 2, the smaller block will start decelerating due to the force of friction with the (also decelerating) larger block. The relevant mass for determining this force is m. The large block will start decelerating due to the force of friction with the road. Note that the relevant mass for determining this force is M+m as long as the smaller block is on top. However, that decelerating force will partly be countered by the smaller block: in conformance with Newton's laws (action = reaction), the same force of friction that pulls on the smaller block towards the left, pulls on the larger block towards the right. So the net force acting on the larger block is the difference between these two forces. Now you can give the equation for the right end of the larger block as a function of time, and likewise for the position of the smaller block, which I guess should be idealized as a point particle with zero width; when the latter exceeds the former, the smaller block will fly off (or fall off) at the right end.
- However, there is a complication. If the velocity of the larger block reaches zero before the smaller block flies off, the larger block will come to a halt, and not start moving in the opposite direction (to the left). Then the smaller block will continue, but the equation for determining the fly-off moment has changed. And, finally, if L is large enough the smaller block may then too come to a halt before it reaches the right end and flies off, and in that case the two will remain together forever.
- I hope I got this right, what with all the interpreting and the complications, and I hope this helps. If you get stuck in solving this, tell us where you got to and how you are stuck, and we'll see if we can help you go on. --Lambiam 11:21, 26 December 2006 (UTC)
Physics
hey are there any shortcuts to be knowing all the stuff your physics teacher knows? any good sites or books? Thanks (sorry but im an ambitious man!) —The preceding unsigned comment was added by 219.78.44.181 (talk • contribs).
- Brain transplantation/Mind transference? (But watch out for Abbie Normal's brain.) Visit Rekall or the teacher on Star Trek?
- Seriously, I don't know how much your physics teacher knows, but if you want to understand physics, then you will have to be willing to invest work in your studies, either now or as a result of accumulated life experience. It will also help you greatly if you already understand basic trigonometry and have studied or are simultaneously studying calculus. It also helps if your mind is oriented towards logic, so after being told about principles "A" and "B", you can generalize and extend that knowledge to principles "C", "D", and "E".
- I really don't think there are any "shortcuts".
- Hi, User 219... I don't have a concrete answer for you, but perhaps checking THIS google result might get you started. Are you looking for info on High School or University level physics? That would help with the answers, to be sure. Good luck! Anchoress 17:27, 26 December 2006 (UTC)
- I dont think there are any shortcuts. Your physics teacher has worked hard to go thro school, taken one or two degrees and put in many hours hard thinking over the years working out the best way to teach physics. The latter part we call experience. There is no real substitue for it. Although you may be able to memorise a load of facts, that does not mean you understand things and without understanding, I think prospects are limited. However, if you purely want to bluff your way through, there are many jokey books, like bluff your wa thro computers etc. Another alternative is to buy a dictionary of physics and learn the words and their meanings. 8-)--Light current 17:57, 26 December 2006 (UTC)
- You mave have heard the phrase: You cant put an old head on young shoulders
- Thats what it means.--Light current 18:00, 26 December 2006 (UTC)
- Try The Physics Classroom, Free High School Science Texts, Textbook Revolution, and Free-Ed.net. The last one has a couple of semesters' worth of university physics lectures on video. BenC7 00:47, 27 December 2006 (UTC)
- There certainly are big shortcuts! I've relied on three of them throughout my entire career. The first one is: search for errors in your textbooks, sensitize yourself to contradictions between separate textbooks, then learn to "un-teach yourself" the information you've found to be wrong. This is incredibly effective because introductory textbooks are full of misconceptions that their authors believe and never question. These misconceptions become barriers which prevent your further learning: like trying to build a brick building on a pile of rocks. So, if you manage to remove the mistakes from your head, you'll quickly gain unusual expertise. You'll learn faster than everyone else because you've defeated barriers which prevent speedly learning in all others who still harbor those misconceptions and can only learn very slowly. Defeating one major misconception is worth weeks of normal study (weeks, if not years!) Yet it doesn't exactly speed up your learning. Instead it lets you learn at the pace which SHOULD be normal, but is actually far faster than the slow crawl of everyone else who has been taught those misconceptions. (Here are a few I've found: http://amasci.com/miscon/)
- Here's another: don't ever try to thoughtlessly "record" or memorize information from books or relayed by teachers. Instead, always assume that books are badly written and full of errors. Try instead to "own" the incoming information by taking it apart and finding alternate ways to describe it to yourself. Be a skeptical textbook editor, not a gullible textbook reader. This does make for very slow going in school, and makes you a "backwards student," but later it allows you to go far beyond all other students. You'll REALLY UNDERSTAND physics, as opposed to just having a head full of disconnected and possibly erroneous facts. And if you're studying on your own, the process becomes easy because there's no classroom pressure to race through the material in lock step with others. I finally heard about another person who learned physics in just this way: Richard Feynman, who said "what I cannot create, I do not understand." That's it! That's exactly it: you cannot memorize someone else's physics explanation, instead you have to disassemble it and then use the parts to construct your own version. This is an ENORMOUS shortcut, since it puts you far ahead of all students who are just trying to memorize facts without understanding, and who will forget all of it after the next exam. Here's another way to say it: it turns you into a physics teacher. You'll never forget any of it no matter how many decades go by. And as you study more physics, it all starts connecting together into a vast mental machine which starts functioning on its own. Those who don't do this, they end up with a big pile of parts inside their heads, but no "machine."
- Here's the third: stop being an egotist. Get into a "zen" state where you don't take your mistakes personally, and where you aren't threatened by information which demonstrates that your current understanding is embarassingly faulty. I stumbled into this particular shortcut by my early religious upbringing which had some eastern concepts. Over decades I saw that many other students were greatly slowed down in learning physics because they had something I didn't: a huge need to protect their egos, protecting their self image of having perfect error-free knowledge. For them, admitting their mistakes and going back to revise their understanding was a huge deal. They'd go into denial and fight fiercely against admitting their errors, fight even more fiercely against ever letting them be discovered by others, and perhaps remain trapped in obvious misconceptions which they couldn't stand to find or fix. Now this would be fine if teachers and textbooks were totally accurate, because in that case our tendency towards errors would be much reduced. If someone needs to be right all the time, there'd be no problem if they actually WERE right all the time. But since teachers and textbooks are extremely imperfect, and misconceptions are the norm, emotional intolerance of personal errors becomes a huge learning barrier. Learning physics involves lots of trial and error, so in a very real sense you have to cultivate a taste for error: to seek out your embarassing personal errors. Do just that, and as with the other shortcuts, you race far ahead of everyone who isn't doing this stuff. --Wjbeaty 05:19, 27 December 2006 (UTC)
I have a friend who manages to get by without knowing or using a single physics formula, by instead substituting purer maths, using imaginary numbers and integration, for example, when working with projectile motion. Seems to work for him --124.243.155.3 09:02, 27 December 2006 (UTC)
- Rather than thinking in terms of 'shortcuts', go to a library and skim a few physics book and see if you can find one that you personally will find the easiest to learn from. Everyone has a different style. Peter Grey 04:32, 29 December 2006 (UTC)
North Atlantic oscillation
Can anyone point me to theories explaining the causes of the North Atlantic oscillation? Thank you! Marco polo 16:20, 26 December 2006 (UTC)
- Brian Fagan, in his book "The Little Ice Age", has a lot of information about it but no clearly defined theories as to ultimate cause, as far as I can see. Geologyguy 16:30, 26 December 2006 (UTC)
- You can find some information on http://www.met.rdg.ac.uk/cag/NAO/Models.html. --Lambiam 00:06, 27 December 2006 (UTC)
Squirrels
We were wondering why a squirrel's tail is so bushy. No doubt there is more than one advantage (and disadvantages), but has science reached a concensus on the primary reason? --Bob K 18:08, 26 December 2006 (UTC)
- Added insulation in cold weather (when squirrels will fold their tails up along their backs, essentially adding another layer of fur). Distortion of their body image as seen by predators (so the predator gets confused and grabs at the tail and not the squirrel's body; you often see "short-tailed" squirrels where this strategy obviously paid off at least once). Perhaps a little bit of aerodynamic resistance (counterbalancing) as the squirrels bound along.
- I thought that the tail also brought some reproductive advantage. For example, I thought that I had read that squirrels prefer to mate with other squirrels with bushy and attractive tails, which are a sign of health and good genes. Marco polo 18:41, 26 December 2006 (UTC)
- Absolutely for balance. Ever seen a squirrel hop from one tree to another? It's quite a feat, and no doubt a tail-less squirrel would be much more prone to plunging to the forest floor — which I have also seen. Vranak
- That explains all of the mass and the flexibility, but none of the bushiness. (Squirrel: It's none of your bushiness.) --Lambiam 21:19, 26 December 2006 (UTC)
- But squirrel tails are not massive! You can see this if you've ever seen a backlighted squirrel: their tails are just like rat tails except for more fur. If they're used as counterbalancing, at least some of the effect must be the aerodynamic resistance of all of that fur in the tail.
- I've noticed that squirrels like to shake their bushy tails at you when they're feeling defensive. The bushier the tail, the more of an impression it makes on interlopers. Vranak
- If they didn't have big bushy tails, they 'd be called rats! 8-)
- Speculating: they might be like the detachable tails of lizards: when a big preditor makes a grab for the squirrel, all it gets is a mouthful of bushy fur. --Wjbeaty 04:38, 27 December 2006 (UTC)
Cation and anions
How do you determine the Cation an Anions of a compund? Dragonfire 734 18:02, 26 December 2006 (UTC)
- If it's a positive ion (missing electrons), it's a cation. If it has extra electrons, it's an anion. --Wooty Woot? contribs 18:19, 26 December 2006 (UTC)
- (Perhaps beating the point to death...) The cations (those missing the electrons) will go towards the cathode, the negatively-charged electrode where they can re-acquire that missing electron. The anions (those with an extra electron), conversely, go towards the anode, the positively-charged electrode where they can give up that extra electron.
- Hmm maybe worth an extra few strokes!
- The term CATion derives from the fact that these ions are attracted to the CAThode (negative electrode) in an electrolytic cell. Cations are therefore positvely charged.
- The term ANion derives from the fact that these ions are attracted to the ANode (positve electrode in the cell). Anions are therefore negatively charged.
--Light current 21:20, 26 December 2006 (UTC).
BUt how do you determine it for example the compound CO2? Dragonfire 734 22:18, 26 December 2006 (UTC)
- What happens when CO2 dissolves in water? You get an acid, right? Which acid? What are the properties of acids in general, and this acid in particular, when it comes to forming ions? --Lambiam 00:02, 27 December 2006 (UTC)
- Well I suggest you look up carbonic acid. THen if you have any further Qs come back here.
heres the dope from that page anyway:
- Carbonic acid has two acidic hydrogens and so two dissociation constants:
- H2CO3 ⇌ HCO3− + H+
- Ka1 = 2.5×10−4 mol/L; pKa1 = 3.60 at 25 °C.
- HCO3− ⇌ CO32− + H+
- Ka2 = 5.61×10−11 mol/L; pKa2 = 10.25 at 25 °C.
If you are looking for a more simple answer, cations are usually the first part of an ionic compound, or generally the metal component of an ionic compound. For example, in NaCl, Na is the cation, and it is also written first and is the metal component of this ionic compound.
Two things the introductory student needs to watch out for: whenever you see NH4, it is almost always going to be a cation; and when you see leading hydrogens in acids, they will always be a cation. The latter statement will need to be explained more.
HCCH is not an acid. HCl is. Both have their first atom as hydrogen, and it is difficult to tell which is which. This is an aspect of chemistry that comes with experience. What you need to ask yourself is if there is any evidence that the compound in question will break into cations or anions. If it does, it is most likely an acid, with H+ as the cation, and everything else in the compound as the anion.--Acewolf359 16:18, 29 December 2006 (UTC)
Classification of an amoeba
I need to know the classification of an ameba thanks Chris H.
- Hi, Chris. Try reading the amoeba article. If you have any further questions, check back here. Anchoress 18:52, 26 December 2006 (UTC)
Bacteria in the Intestines
The large intestine hosts several kinds of bacteria that deal with molecules the human body is not able to breakdown itself. This is an example of symbiosis.
We can be fairly certain that if we were able to hypothetically remove, all at once, every bit of the bacteria from the intestines of the average human being, the bacteria itself would almost certainly perish without its host. But what about the human? Would they be likely to perish as well, in the complete absence of this intestinal bacteria?
Have the two systems developed complete codependency? Are many of the normal flora present in our bodies likely to belong to similar codependent relationships? And I believe that pregnant mothers pass down their bacteria to the fetus. Is this correct?
Thanks ahead of time...71.246.38.187 21:34, 26 December 2006 (UT
- I imagine the substances (such as cellulose) would simply be excreted with feces. This might prove a problem with excessively large or pointy undigestible material, but by the time it gets to the large intestine it's probably done its damage anyway. --Wooty Woot? contribs 03:16, 27 December 2006 (UTC)
Gut bacteria are, in fact, quite important for our health. Even besides the assistance in various digestive tasks, including absorbtion of broken down materials, they also produce much of our vitamin K2, without which we are unable to perform such vital tasks as clotting. We would live without them, very likely, but it wouldn't be an especially pleasant life. Leave your bacteria alone, they are your little, little friends. – ClockworkSoul 03:53, 27 December 2006 (UTC)
- I beleive dysentery has the undesirable effect of killing most of the bacteria in the gut.(although our page doesnt say so)--Light current 06:10, 27 December 2006 (UTC)
- Oral antibiotics commonly do have that bad effect as well.
- Atlant 12:44, 27 December 2006 (UTC)
- The immediate problem with the use of broad spectrum antibiotics is that they don't kill all the bacteria, leading to overgrowth by species usually kept "in line" by the others, with the possible result of C. difficile enterocolitis, toxic megacolon, and death. - Nunh-huh 13:11, 27 December 2006 (UTC)
- Atlant 12:44, 27 December 2006 (UTC)
Paternal Age
Paternal Age ==
Might Misplaced Pages be interested in my research on the connection between advancing paternal age and sporadic cases of these conditions: Ihave found research papers on the following conditions all finding increased incidence with advancing paternal age
Multiple Sclerosis, Diabetes Type 1, Athoid/dystonic cerebral palsy, hemiplegia, Acute Lymphositic Leukemia, pre-menopausal breast cancer, some Alzheimer's, shorter life for women (fathers 45+), prostate cancer, epilepsy, some early childhood cancers, schizophrenia, autism, Multiple edocrine neoplasia type 2B, Hemophila A X linked maternal grandfather, progeria, marfans, achrondroplasia, aperts, some heart defects, other very rare disorders, Down Syndrome if the mother was 35 or older and the father was 40 or older if this information can be used by someone for an entry or for inclusion with entries already in wikipedia I would be happy to give the citationsAnniepema 21:53, 26 December 2006 (UTC)
- Misplaced Pages has Maternal age effect.....I suspect we are rather weak on this topic. --JWSchmidt 00:59, 25 December 2006 (UTC)
- There has been a recent spate of inclusions/reversions on this topic on the menstrual cycle page, perhaps you should check there to see if your contributions would help. Anchoress 04:41, 25 December 2006 (UTC)
Anchoress maybe you didn't read that I was asking about interest in paternal age. The paternal age effect has to do with ,for instance, the ground-breaking work of NP Singh et.al. from the Unversity of Washington in Seattle in the research paper "Effect of age on DNA double-strand breaks and apoptosis in human sperm" In this research it was found that the percentage of sperm with highly damaged DNA and DNA break numbers was statistically significantly higher in men aged 36-57 than in those aged 20-35 years, but percentage apoptosis was statistically significantly lower in the older group. This finding was the first to suggest that there was an age related decrease in human sperm apoptosis. This new finding may indicate deterioration of the healthy sperm cell selection process with age. Also the work of James F. Crow on the high rate of spontaneous mutation in the sperm which increases with age is relevant and Andrew Wyrobeck's research on sperm DNA damage with age.. Anchoress I think you read the last part about the maternal age effect and not my question about if there was any interest research on paternal age Anniepema 21:51, 26 December 2006 (UTC)
- No, I read exactly what you were asking about, and as I said, there was just an inclusion on the menstrual cycle page about that exact topic; interestingly enough - since you have expressed an interest in providing references - asking for a reference. I'm curious to know why you thought I had mis-read your question? Anchoress 08:24, 25 December 2006 (UTC)
- I wouldn't have expected menstruation to be dependent on paternal age, myself, but perhaps I'm wrong. StuRat 11:13, 25 December 2006 (UTC)
As for an article on paternal age, sure, that would be great. However, since Misplaced Pages has a "no original research" rule, you need to only use sources in published works. If your own research is published, that's fine to use, but you can't include info from any unpublished studies you've done. StuRat 11:13, 25 December 2006 (UTC)
Sorry Anchoress
I didn't understand that the statement about male sperm be freshly made was what you meant by the new inclusion. The opposite argument is made about the many genetic diseases associated with older paternal age at the time of birth because of all the cell divisions to make new sperm.
Excerpts from geneticist James F. Crow's "The High Rate of Spontaneous Mutation:Is it a health risk" PNAS August 1997
"Paternal Age Effect"
"How can we account for a higher mutation rate in males than in females? The most obvious explanation lies in the much greater number of cell divisions in the male germ line than in the female germ line. In the female the germ cell divisions stop by the time of birth and meiosis is completed only when an egg matures. In the male, cell divisions are continuous and many divisions have occurred before a sperm is produced. If mutation is associated with cell division, as if mutations were replication errors, we should expect a much higher mutation rate in males than in females. "At age 20 the number of(sperm) cell divisions is about 200, at age 30 it is 430, and at age 45, 770."
This makes the strong prediction that the mutation rate should increase with the age of the father, since the older the man, the more cell divisions have occurred. On the other hand, there should be no age effect in females."
Let me interject at this point that there is a well-known maternal age effect for traits that are caused by errors in chromosome transmission. The kind of accident that leads to a child with an extra chromosome is strongly associated with the mother's age (15). There may be a slight paternal age effect, but the far more striking effect is maternal. My concern, however, is with gene mutations which, when those with small effects are considered, are much more frequent." .... Anniepema
Can anyone write any kind of acceptable entry in wikipedia on the paternal age effect that is well written and neutral?Anniepema 06:34, 27 December 2006 (UTC)!Anniepema
December 27
Lack of Visual 'Imagination'
I am unable to "visualise" anything. If I close my eyes, I see only the back of my eyelids and have never been able to summon an image.
And whilst I very rarely dream (or remember them as I'm sure people will claim), the very few I recall having have not had any pictures, but more like reading a story -- I know what is happening but not in pictures.
This has always been the case, though I only realised when I was a teenager that when people said "visualise" they were being literal and could actually see pictures in their head!
My question, is there a name that describes this psychopathology, and is it a common thing? Any studies regarding this and how it relates to creativity and inteligence? Is it perhaps an autistic spectrum disorder?
This facet of myself does not worry me and any information will not be taken in any way as medical advice. So any pedants can please leave other more useful responders free to speculate.
86.132.225.66 00:35, 27 December 2006 (UTC)
- Who knows — maybe dreaming is more 'pathological' than not dreaming! At any rate, I do not recall learning about this or any similar condition while studying psychology.
- Dreams serve to enlighten and evaluate, so perhaps a lack of dreaming, or lack of visual imagination, is a good sign that you are already quite enlightened, open-minded, free of social bias, and so on. Vranak
- I've had colleagues who were not "visual thinkers," and their understanding of science was entirely verbal. Perhaps they lacked the ability to see pictures in their heads? By analogy, I've heard about people who can play actual music in their heads, whereas I'm more limited and can only remember how a tune goes but not hear instruments playing it. I'm definitely a visual thinker, but when I see a picture in my head, it's nothing like an image on my retina. It's more like a memory. If I visualize a dark background with a square made of glowing green lines, I can't exactly see it, instead it's more as if I'm remembering what such a vision looks like. I can make this green memory-square shrink and grow, rotate, change colors, etc. But it's only as detailed as a fuzzy memory, and if I try to add too many details to this vision, I'll lose track of some of them. On the other hand, I've heard of visual thinkers whose internal vision is just as accurate as reality, and who can build incredibly detailed objects and then continue to "see" them. If visual thinking can range up to that high ability, then it probably ranges down to where it's so fuzzy that it becomes useless. --Wjbeaty 04:36, 27 December 2006 (UTC)
- Although "imageless dreams" are regularly reported, this is the first time I hear of someone who otherwise has normal vision only having such dreams. Most people report having at least occasionally dreams that are visually as vivid as they experience the world when awake. In contrast, visual imagination is normally not reported as having a quality of vividness, in accordance with Wjbeaty's comment, and neither is visual recall of a familiar image. From the scant reports on people with vivid visualization, it is not clear whether this is coupled for imagination and recall. The usual lack of vividness of recall is in marked contrast with the ability of many people to have a vivid recall – often involuntarily – of sounds and in particular melodies. But most people are unable to "imagine" a new melody. Apparently these various abilities are not tightly coupled. --Lambiam 10:15, 27 December 2006 (UTC)
- If you are really unable to visualize at all, that would be very interesting from a cognitive perspective. You might offer yourself up to a university for some MRI scans — I have a feeling cog sci graduate students would be very interested in seeing how your brain looks, whether or not the visual centers look the same as other people's. Because even blind people can usually visualize, because they still have the same brain hardware as the sighted. It's very, very interesting that you cannot, though you can still see fine? It is very curious. --24.147.86.187 14:24, 27 December 2006 (UTC)
- This is why I was asking, if it is a recorded phenomena then perhaps there is some research into it, and it has a name. Alternatively perhaps there is a spectrum between people who can see images as clearly as if they had their eyes open (which I've heard people claim), and as Wjbeaty said down to a fuzzy near useless level. Could anyone tell me the field of study this is? Or even the name for this sort of ability, "imagination" sounds rather vague.
- I also can not hear sounds either (unless from my ears), but this seems more common. Some people, musicians for example, do claim they can hear a tune in their heads.
- Another example from my strange mind :) I am reasonably poor at recognising faces (this does have a name which escapes me at the minute, but I'm not incapable in any case). If you asked me to describe someone I can't "see" them, but I just "know" that "A has short brown hair, big nose, and pointy eyebrows", as if I had read it some where, and if I want to feel confident about recognising people in future I will consciously remember these features as a list like that.
- Incidentally, I did have, 17 or so years ago when I was 8 or 9, CAT scans and EEGs looking for something I wasn't clear about. I was a very quiet child and I think Autism was suspected, but nothing conclusive was found AFAIK, and doubt anyone would now think I was Autistic. 86.133.203.195 00:11, 28 December 2006 (UTC)
- Did you try painting the back of your eyelids - if not, just imagine! -- DLL 18:31, 27 December 2006 (UTC)
- 86.133.203.195, can you draw a room that you're not in right now? Is your drawing accurate? If so, you can visualize things, because there's no way you can make an accurate drawing using only a worded description. --Bowlhover 07:50, 28 December 2006 (UTC)
- To expand on the previous comment, can you draw a room that you are not in currently? Also, have you ever hallocinated? You may want to try going into a sensory deprovation chamber.--Acewolf359 16:23, 29 December 2006 (UTC)
Wow, looks like it has been sometime since anyone looked into this. I however am very interested as I am in the same situation. I did not realize people could acutally 'visualise or picture' anything, I thought it was just a term people used, I just asked my husband if he could actually see things last year (I'm 36). I was very surprised to find out that people can actually SEE things, my son claims he can even see with his eye's open and makes a game of it sometimes when he is bored. I do however see images in my dreams. I have not been in a sensory deprovation chamber however I have sat in a dark room on a winter night where there was nothing to been seen or heard and all I saw was black. I can draw a room for you but it is not being seen by me I just remember where items are at, it is more like someone is reading the information to me. Is there a term for this? Thanks- Tink
Is red and pink coral illegal in Canada?
Hello,
I recently was caught by surprise at seeing some beautiful red coral and silver jewellry pieces in a local ethnic gift shop. I have never seen red coral jewellry before in Canada (but I haven't searched extensively!), and for some reason I have always thought it was illegal. Upon questioning the salesclerk, she answered that it was not illegal as the coral was already dead before it was obtained for use in making the jewellry.
1. Are products made from red coral illegal in Canada? 2. Is one being ecologically ignorant in wearing jewellry with red coral?
Thank you! 74.115.30.54 01:11, 27 December 2006 (UTC)
- First, the argument that "it was already dead before it was obtained" is bogus. If the store sold you something that was illegal, the next thing they'd do is order another one, which someone would kill, and then it too would be "already dead". The idea of such prohibitions is to stop the dealing in such products in order to remove the motivation for the killing.
- It appears that there is no specifically Canadian law about coral products. Canadian federal laws can be read and searched at the Department of Justice web site, and their search finds nothing containing the word "coral". However, that doesn't make it legal. Canada is a signatory to the Convention on International Trade in Endangered Species, and several species of corals are regulated under this treaty. I have no idea whether there are any red corals that do not fall under CITES and whose sale is therefore legal.
- --Anonymous, December 27, 02:18 (UTC).
- I assume the clerk meant that the coral had died, not from the actions of those collecting it, but from something else previous to that time, like pollution, for example. I believe tusks collected from elephants which died of natural causes can also be sold legally in some places, but there is a certification process used to prove that this is, in fact, the case. I'd expect something similar for coral. On the plus side, such a program may serve to satiate the demand, thus reducing poaching. StuRat 13:26, 27 December 2006 (UTC)
- I don't know anything about why red/pink coral may be illegal in Canada, but why must you be ecologically ignorant from wearing coral? Endangered species in the United States can often be found to be plentiful in one area, and almost gone in the other (thus they are endangered, there). What if red coral is plentiful some other place in the world? Just being skeptical! It is my understanding that coral is fairly plentiful, and not that tough an organism (small salinity changes or temperature changes, or water current changes can severly stress it), even though the volume makes up for it. Correct me if I'm wrong about that. X (DESK|How's my driving?) 22:39, 27 December 2006 (UTC)
Canada's Species at Risk Act / COSEWIC mainly deal with Canadian species. However the CITES convention, which Canada has ratified in 1975, lists two coral genuses in Appendix II, meaning trade is restricted because they may become threatened by trade (although not currently threatened) or look like threatened species: Fire corals (Milleporidae species), and Lace corals (Stylasteridae species). So you probably don't want to be doing trade in corals of those genuses cause they would be bad. Judging from the name, fire corals are probably red. Note: fossils are not subject to the provisions of the Convention. —Pengo 06:45, 28 December 2006 (UTC)
- Also I don't recall ever seeing a dead coral that still has its colour. Is it possible that the coral was dyed? —Pengo 06:56, 28 December 2006 (UTC)
Many countries whose waters contain living reefs have laws regarding the harvesting of coral, (of any color), and even stronger laws regarding it's removal from the country. Coral that has any color left in it was harvested directly from the reef or found very soon after a trauma, (boat, diver, storm, etc...). This doesn't necessarily add up to illegal coral, if it is in fact real coral at all, but it's not likely to be on the up and up.Oons
skins compression clothing
I was wondering what effects the use of skins compression clothing have on athletes in terms of both performance and recovery. I was also wondering what is the difference between skins and other brands on the market
muscles
Is it harder for asian males to get lean muscles through weight-lifting compared to other races?
- I don't know an answer, but this is related to somatotypes. I do not believe one can look at it by race very well, however it is possible that different somatotypes are found in different magnitudes in different races? Many scientists argue the existance of "races." Don't worry, we still believe in marathons. X (DESK|How's my driving?) 22:34, 27 December 2006 (UTC)
Science name
What is the name of the science that deals with the size and shape of the Earth? My sister got asked this, and didn't know. I'm just interested! --Thelb 09:04, 27 December 2006 (UTC)
- Planetary science (aka planetology)? Clarityfiend 09:13, 27 December 2006 (UTC)
- Geology ? (or surely one of the many links at the end of the article) -- WikiCheng | Talk 10:01, 27 December 2006 (UTC)
- Geodesy? Weregerbil 10:48, 27 December 2006 (UTC)
- Ding! Ding! Ding! We have a winner. Clarityfiend 11:29, 27 December 2006 (UTC)
What was a man able to withstand of wounds and injuries during the past (medieval times)
hey there 'Misplaced Pages-people', your doing impressively well with this site. I love it =D
I have questions about how much would a man be able to withstand in the past when it came to wounds and injuries from melee-weapons and effects from extreme cold in the medieval age.
i realize that what i am about to ask might seem very strange questions, so to take away any curiousity around it if any, I just say i'm writing a book/novel and so i need facts about this and that, and i use Misplaced Pages a lot as a source of information, and i realize my questions may not have direct answers, but rather 'guiding' answers that points me in the right direction. If you have any idea at all, that is.
1) A man who takes a axe-cut across for example his hip-bone so strong that his hip-bone are broken/crushed and that his skin and flesh are sliced open to a big open wound, blood flowing out of it quite good, would he stand any chance of survival ? in the past, i take take it they had no methods of 'fixing' broken body parts, like we have today when we go through surgery and such. but would he die in just minutes, or would he die a slow lingering death that stretched for over days ? Would he fade slowly and eventually 'fall to sleep' relatively peaceful due to blood-loss or would there be chances that he died by the injuries itself first ? or would it be chances to save him ?
2) Say that this axe-wound was made out in the wild, in arctic, cold and harsh lands far away from civilization. If there were forexample -15 degrees or even colder, would the cold have ANY impact on 'freezing' the wound and stopping the bleeding on such a big wound ? any impact at all? if any, then HOW strong impact? would it help keep pressing cold snow over the wound ?
3) how big and bad could a wound be before the cold no longer could HELP stop the bleeding from it?
4) what methods did they normally use to treat battle-wounds and stop bleeding ? forexample a an arrow-wound or a lesser sword-sting or lesser sword-slash?
5) If one falls through the ice and into the water, and then gets up on land relatively quickly how long would you be likely to survive in the wild with temperatures as low as -15 or lower? a few minutes, right ? ofcourse, one would have to tear of all clothes in a hurry, as the cold and wet clothes will only add to make you frozen. But then what ?
6) was it possible that a bludgeon-weapon (war-hammer, mace, morning-star, flail, club etc.) could kill a man THROUGH leather, mail or steel-armor in ONE blow ?
if these questions fits better under "culture", since its from the past, just say and i'll put it there instead, but here stood "physique" so...
thank you, Krikkert7 10:58, 27 December 2006 (UTC) Krikkert7
- I can't answer all your questions, but I can answer some. First, people in olden times have survived remarkable injuries; Samuel Pepys had a gallstone taken out without any long-term ill-effects; the son of a mediaeval king (sorry don't remember his name) had an arrow painstakingly removed from - I think his head - and lived, but of course, on the flip side, many many people died of injuries that would be easily treatable today. I think it was King Richard the Lionhearted who died of a fairly minor (well, serious but not mortal) shoulder injury.
- As to your first question, even nowadays with fast and efficient medical care, people die of injuries to the femur, even with the skin unbroken; it would be unlikely that your medieaval hero would survive. The speed of his death would depend on whether or not the femoral artery was nicked or collapsed as a result of the injury; if yes, death could be in 10s of minutes or sooner; if no, then he would die of gangrene or shock.
- As to cold and injury, cold is seldom good for any serious injury, particularly those that involve blood loss, because it hastens shock, the depression of the cardio-pulmonary system, blood flow and blood pressure that results in unconsciousness, heart failure, organ failure, and ultimately death. The only good field treatment for serious, bloodletting injury is compression, and I am sure many mediaeval warriors knew this.
- I hope others can expand upon my answers and answer your other questions. Anchoress 11:19, 27 December 2006 (UTC)
- Interesting questions. I'll try to give some answers. Know this is just speculation, (although intelligent speculation i'd hope).
- 1. The person would probably faint from the pain. The most immediate way this man would die is from blood loss. An axe-cut that deep would cause a LOT of blood loss, with the person dying in minutes-hours depending on the exact location of the cut. The Axe Cut could easily have damaged his internal organs, or the broken bone could have stabbed up and damaged his internal organs. The next biggest concern is infection. If he doesn't die immediately, the wound will most likely be infected. There're ways to prevent infection (salt, alcohol, burning), but none of these are all that reliable. With no antibiotices, he'll die in days-weeks depending on how big the wound is. So for chances of survival - i'd say very low. Although it does depends on where exactly the wound is and how serious it is. Even if the man miraculously survived on his own, you can be sure he'll never be able to walk or even stand again with a hip bone that badly damaged. Even so, he probably won't survive more than a few years.
- 2. I'd say the person would most defintely die in those conditions. The cold would 'freeze' the wound, stopping bleeding and halting infection. But...-15 degrees is VERY cold. THe axe cut would also rip of clothing, exposing not only the wound but the entire body to the cold. Being exposed to -15 degree whether causes a whole lot of problems, as Anchoress has already described above.
- 3. depends on how cold. If we're talking -15 degrees...i'd say the blood would freeze pretty quick - no matter how big/bad the wound is. But in that kind of temperature, the person would die anyway from the cold.
- 4. you mean with modern medicine? Well...i don't think many people get such battle wounds these days. I'd guess they'd deal with arrow-wounds the way they deal with bullet wounds (so surgically remove the arrow, stop bleeding, antibiotics to prevent infection, stitching...etc.). Back in the medieval times...dunno. HIstory was never my thing. But i'd guess it'd be just a matter of pulling out the arrow, and bandaging it and hope it stops bleeding. If the wound was very bad, they'd probably use this technique that involves basically burning the wound. There's a special word for it but i forgot. I'm not sure how it works, but it's supposed to stop bleeding. Salt/alcohol/heat would be used to kill bacteria, and hence prevent infection. Then the person would just have to rest, and hope his body can make it.
- 5. if the water was -15, then someone falling into it won't be able to climb out. It'd send you into shock - both phyiscally and mentally. You try jumping into a pool of cold water on a cold day without preperation, and see how quickly you can get yourself out. plus, climbing out of the ice is not easy. That's why so many people die from skating on frozen lakes/rivers - because they fall in and can't get out. I mean, if you fall through the ice, you'd be falling through a small hole. Climbing out is not so easy. Just think about how you climb out of a swimming poll without using the ladders. Then imagine doing that in -15 degree water, with slippery ice. And...can this guy even swim?
- 6. yeah. If you hit hard enough and at a vital spot. You get hit on the neck/head with a bludgeon-weapon that's swung right...and you'll die. Leather doesn't offer much protection. Not sure about mail or steel armor. But from my understanding, armor protects more against slashing weapons. it's like...when a bludgeon weapon hits, the force of the blow will push you back regardless of armor. So if it's swung with enough force, i don't see why it wouldn't still be able to damage/kill. I doubt the armor can absorb that much shock - a clear hit would kill the person, especially if it's in a vital spot.
The biggest difference between then and now is the risk of dying from infection. They would have been perfectly capable of preventing most infections, by cleaning the wound (say by pouring whiskey on it) and by only wrapping in sterile cloth (say by boiling the cloth), but didn't know that wrapping the wound in old dirty rags would cause an infection, so were likely to do more harm than good. StuRat 13:20, 27 December 2006 (UTC)
- If the water was -15 degrees (F or C) it would be frozen solid, so he would not fall into it. (perhaps onto it). People up until the 1930's when sulfa drugs were introduced and the 1940's when Penicillin was introduced often died of very minor infections, despite the best medical care. The son of President Calvin Coolidge got a small blister from a tennis shoe which got infected and he died despite all available medical science. Yet a few hardy souls survived amputations, penetrating wounds of the head in which a steel bar passed all the way through the brain, Phineas Gage (1848) and wounds which left an open fistula into the stomach Alexis St. Martin (1822). Edison 17:19, 27 December 2006 (UTC)
- For the record Pepys' surgery continued to degrade progressively untill he died and may have had something to do with it. 68.39.174.238 04:55, 2 January 2006 (UTC)
What was a man able to withstand of wounds and injuries during the past (medieval times) - PART 2
Hey, THANKS LOTS FOR UR ANSWERS on my previous questions!!! ...but now you got me going for more with all those good answers :D :P
Ur very helpful to me!
7) As for the the guy falling in the water, i was thinking more like he got help from some mate/friend who drags him up quickly. i understand he would still go in shock, and die HOW FAST??? how would the process go about ? would he sit quite unmovable, shaking ? not able to speak, and in shock as said? and eventually his body would not be able to take it anymore, and he would FADE SLOWLY ? or would it all happen more quick and brutal ? i guess his friend would do him a favour by ending his misery quick... :S
8) And quite interesting i think, how would this guy who pulled him up fare ? how would his arm and hand fare ? (Ofc, his hand has to be put into the icy water and get wet to pull his friend up - say he's quite strong and able to do so with only one arm) I take it chances would be BIG for him to be hit by frostbite, or maybe he would be WITHOUT ANY DOUBT in such a harsh climate? At least his fingers and hand would be greatly in risk, no ? However, if he quickly dry the arm and hand as best he can with something dry and then wraps arm/hand in as much cloth he can to generate warmth, he may be able to "recover", no ? - even though they are out in the wild with extreme cold...
9) And.. as I know, if frostbite gets to bad, and gets infected or is hit by gangrene, one has to amputate the limb/bodypart that is infected. i tried read about amputation ( in the past) but found nothing about how they stop the bleeding... i mean, if u forexample saw of someone's leg or arm, HOW ON GODS GREEN EARTH are you going to be able to stop the bleeding from such a big open wound? the blood must flow out in less than a minute ?!?! :O i read scarcely that they lingate the main arteries and veins BEFORE the 'operation', but they didnt do that in the past did they ? and how would they be able to do that before they had sawn the leg/arm of ?
10)you say extreme cold is bad for any big wound, and i guess i see the logic in it. the cold goes straight INTO your body through that huge open wound i guess and chills down/cools down and kind of freezes your INSIDE, affecting inner organs and all that much much faster. but you did not give me an opinion on how fast he would die when counting'in the extreme cold. so... ?
thank you Krikkert7 14:48, 27 December 2006 (UTC) Krikkert7
Lipitor and grapefruit
How dangerous is eating a daily grapefruit while on the lowest dose of Lipitor?
- We really can't tell you how dangerous it is, but you should avoid it, as grapefruit causes many meds to stay in the system longer than usual and thus have more of an effect than usual. Of course, if this effect was predictable, just reducing the dosage of Lipitor would solve the problem. Unfortunately, I don't think anyone has studied it to know exactly what the magnification factor on Lipitor is, so any reduction in dosage would be little more than a guess, therefore I would just avoid grapefruit, instead (which is a shame, because grapefruit is really good for you). Perhaps we can get the gov to fund a university study ? StuRat 13:07, 27 December 2006 (UTC)
- There have been many such studies. Lilja JJ, and co-investigators ( Clin Pharmacol Ther 1999; 66: 118-27) found that grapefruit juice increased Lipitor's availabilty by about 2.5 fold. Another, Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin.Br J Clin Pharmacol. 2005 Nov;60(5):494-7 Ando H, et.al, found that grapefruit juice increased the mean AUC(0-24) of atorvastatin acid by 83% (95% CI 23-144%). The problem is that such studies do not predict the magnitude of the effect in any given person. So that even were someone able to keep their daily grapefruit juice intake constant, they would need individual testing for blood levels of atorvastatin and its metabolites to determine its effect. That's just not practical, and it's much easier just to avoid the grapefruit. - Nunh-huh 13:22, 27 December 2006 (UTC)
- See also Cytochrome P450 oxidase, the specific enzyme pathway affected.
- The effect is variable, as grapefruits have widely differing concentrations of the relevant bioactive compounds, and therefore not usable in a reliably predictable way. --Lambiam 15:45, 27 December 2006 (UTC)
Nuclear war
What effects would a full-scale nuclear war have on humanity?
Obviously they'd be negative, but as for the scale of the destruction, I can't get a clear answer. I mean, take a look at pop culture:
- On The Beach - All human life is completely wiped out, even though no nuclear weapons were deployed in the southern hemisphere
- The Outward Urge - The northern hemisphere is reduced to a poison wasteland, the southern hemisphere survives and thrives.
- Threads - Life survives even in the worst hit areas. Of course, it's not a good life.
- Jericho - You can live right in the heartland of America and everything will be fine! :)
Which is the closest to the truth? Battle Ape 13:57, 27 December 2006 (UTC)
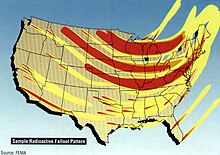
- Most scientists don't think nuclear winter would occur, but that isn't much of a consolation. Aside from having most large population centers destroyed, the nuclear fallout would deposit lots of very nasty radioactive poisons over most of the rest of the world as well, as well as raise the level of background radiation considerably. Could humanity survive? Maybe, but it would be a very different sort of survival than we have now. Would civilization survive? Probably not as we know it. Oy. In any case we have an article on nuclear warfare which might be helpful. Of the above choices, I'd go with Threads. --24.147.86.187 14:14, 27 December 2006 (UTC)
- One effect that is often under-considered (at least in popular media) is the effect on the world's economic systems. The 9/11 attacks, an attack which was relatively small-scale as war-making goes, had a vastly-disproportionate effect on morale in America (and to a lesser extent), worldwide and this propagated into substantial downturns in the US and global economies. You can bet that after a full-scale nuclear exchange, there just aren't going to be that many people buying (or manufacturing) large-screen plasma TVs; we'll be lucky if the world doesn't starve to death from lack of global trade in food. Also not inconsiderable will be the fact that a full-scale exchange will remove many of the cities that are home to the world's financial systems. It's a sure bet that the aftermath won't be pretty, and Dr. Steven Falken's strategy (of living quite near a primary target site) may well look to have been quite appealling in retrospect.
- Atlant 14:27, 27 December 2006 (UTC)
- Not just starve to death, but all of those bodies left to rot because there are not enough people around to bury them, or even care to do so, will be cess pools of disease just waiting for the next unwary traveller who passes by (or, who, starving, decides to eat the dead, or dying, corpse). User:Zoe|(talk) 17:54, 27 December 2006 (UTC)
- Atlant 14:27, 27 December 2006 (UTC)
- Most of the population is in the cities, and those would be the prime targets. Thus most of the people would be eliminated, which is lot's fewer mouths to feed. So starvation? Who knows, certianly an overload of canned food would be available, and the can would protect the food inside from fallout. Would there be too many people left to live off the land after the radiation subsided? Who knows, and it depends a lot on the type of nuclear war, how many 'salted' nuclear weapons would be used? etc. 12.10.127.58 22:36, 27 December 2006 (UTC)
- Most of the population is in the cities...
- Citation for that? Although I suppose it depends on what you define as a city elligible of being on the receiving end of a nuke or ten.
- According to the World Almanac, using US 2000 census data, 226 million (80.3%) lived in metropolitan areas. About 135 million lived in the 25 largest metro areas. Cheers Geologyguy 22:57, 27 December 2006 (UTC)
- Which still, unfortunately, leaves open the question "in a metro area worthy of a bomb?" Remember, lots of them will be expended over-targetting military installations.
- The fallout map above shows the targetting of only military installations. Most major metro areas would be covered in very high levels of fallout even if they weren't targetted, and many major metro areas are the sites of military installations or have military installations right next to them. "Just" targeting the military sites doesn't really get one off the hook. --24.147.86.187 14:05, 28 December 2006 (UTC)
- I'd imagine that the human race would survive, even if 'civilization' as we know it didn't. There are plenty of 'crazy survivalist'-type people who prepare for the eventuality of nuclear war on a daily basis - perhaps one day they'll be the ones smiling as they emerge from their purpose-built, isolated, well-supplied underground shelters after a few months waiting for the radiation levels to decrease somewhat. The world afterwards will not be pretty - those that survived the war and the radiation will probably have to live through decades of doing their best just to survive and keeping their heads down whilst various factions fight amongst themselves for control of what's left. With no power structures left in place (how long do you reckon that the politicians that were responsible for the war would survive before being lynched once they came up for air?), there'll be plenty waiting to fill the vacuum, be it 'to rebuild the earth' or simply for their own ends. Imagine a world where anyone with leadership qualities and a political opinion gets to voice it with an army of gun-toting followers behind - a complete and utter free-for-all. --Kurt Shaped Box 01:51, 28 December 2006 (UTC)
- Thanks for the answers (that map is odd though; that's one big-ass nuke that landed in Montana), but I'm specifically wondering about other countries. Obviously the USA would be a ruin, along with Russia or China or whoever started it, and probably Europe. What about lesser countries, say, Australia or New Zealand or Chile? There would obviously be economic collapse, but would they be free of fallout? Battle Ape 01:47, 28 December 2006 (UTC)
- It's not one big nuke in Montana, it is an attack against the Minuteman missile fields which are/were in Montana. The map dates from the late 1980s so many of the sites have probably been decommissioned by now, though. As for countries in the Southern hemisphere — they would probably not suffer much direct fallout, though the effects on worldwide background radiation levels from a full nuclear attack would raise considerably, and it would definitely show up in their food supply over time. I'm not sure what the long-term effects of that would be but you can imagine quite plausibly a much increased level of many cancers, for example. --24.147.86.187 14:05, 28 December 2006 (UTC)
Beyond the four stories you cited at the beginning ofthis thread, you might want to see List of apocalyptic and post-apocalyptic fiction -- there are lot's of "after the next war" stories listed there.
Atlant 12:42, 28 December 2006 (UTC)
For a pretty good example of how people manage to survive an apocalypse, may I recommend Dies the Fire by S. M. Stirling? User:Zoe|(talk) 22:15, 28 December 2006 (UTC)
Related question: What the heck is in the back end of Nebraska? 68.39.174.238 05:45, 2 January 2006 (UTC)
- Many megatons of nuclear destruction, just waiting in their ICBM silos.
I'm sure the human species would not be wiped out. Even a nuclear winter would be almost certain to leave some survivors (probably in the high Arctic where the ecosystem has adapted to months of constant darkness, the southern tropical rainforests , and any prepared shelters.). If there were no nuclear winter, I doubt if people in, say, Uruguay or Papua New Guinea would know anything had changed, except through the media. (There's a Ray Bradbury story about this, where a Mexican farmer watches US people flee south to avoid the final war, then shrugs and goes back to his work - it won't affect him.)
The human race has survived disasters as bad as a nuclear war would be likely to be, when we had much fewer people and resources and much less information. There was an eruption at Lake Toba during the Paleolithic which had, accroding to our article, the energy of a gigaton of TNT. That's 1,000 megatons (for comparison, Little Boy was about 15 kilotons, and the largest hydrogen bomb ever exploded was 51 megatons). Though there was no fallout, it may have caused global cooling and a nuclear-winter-type effect. Vultur
Highly unlikely that all humans would be killed.
Much much much more unlikely that all life could be wiped out. Bacteria can survive for thousands of years buried miles under ground or ice, next to boiling water, etc. If the moon collided into the Earth? Maybe, but I doubt it even then. — Omegatron 22:10, 3 January 2006 (UTC)
Tracking of dynamically-changing objects on TV
I am searching for an algorithm (or an approach) which could help track identified (outlined) objects seen on TV set. Say, if I capture a TV broadcast to a medium (tape, hard disk of a computer, etc.), is there a solution to help identify (maybe outline) an object on the screen, and then have it "automatically" tracked?
An object on TV (raster or bit-mapped) typically has the following characteristics: Its shape changes. IT appears multiple times (imagine a ball passing behind a player momentarily in a sports broadcast). Its trajectory changes. Depending on lighting and reflections, its color is not consistent, etc. Any suggestion is welcomed: An approach, a particular field of study, one or more algorithms dealing with raster-based or bit-mapped graphics.
Where should I start? Where should I research for an answer?
If you cannot think of a specific answer, is there a particular area of research or commerce I should focus on?
Thank you. I look forward to your creative suggestions and guidance!
Kelar12 14:42, 27 December 2006 (UTC) KT
- The article on video tracking may be of help. - Dammit 14:55, 27 December 2006 (UTC)
- Try edge detection. That's on what most of the visual tracking algorithms are based. --V. Szabolcs 22:59, 27 December 2006 (UTC)
Once you detect the object in two frames, you can calculate approximate velocity and predict which other locations to look for the object in other frames. As the object may be accelerating or decelerating, this velocity calc should be refined for each additional sighting of the object (adding a new "data point" to the displacement curve). Sightings which fall well outside of the expected location should be ignored, such as if a second, identical ball appears on the screen. This method would also allow you to plot the probably location when the object is hidden. StuRat 01:48, 28 December 2006 (UTC)
Thank you. I appreciate the feedback. Keep any new suggestions coming! Kelar12 06:39, 28 December 2006 (UTC)
- 2D cross-correlation of pixel values of subsequent frames?
- Match moving is pretty related. — Omegatron 22:00, 2 January 2006 (UTC)
Note that the apparent size may also change, as the object moves closer or farther from the camera, and the shape may "morph", as it rotates. StuRat 20:13, 28 December 2006 (UTC)
This probably won't help you if you don't have access to the broadcasts, but cricket television coverage in Australia has a feature which will show the supposed trajectory of a bowled cricket ball if it wasn't blocked by a bat or leg - used in controversial lbw decisions. This is a lot simpler than tracking the ball after it was hit (for example) or other sports like football or tennis where the ball could go in any direction. If you aren't in a cricketing nation, then check your local cable TV or web stream to see what I mean. (Looking on youtube now, but can't find anything!) -- Chuq 10:59, 30 December 2006 (UTC)
Fatty Acid Enzymes
How do the desaturase and elongase enzymes that operate on fatty acids know which types of fats to act upon? For example, why do enzymes like delta-6-desaturase "know" to operate primarily on EFAs, and not saturated or monounsaturated fats? Frankg 15:02, 27 December 2006 (UTC)
- Enzymes have active sites that are very specific for their substrates and can distinguish between the different structures of the fats. David D. (Talk) 17:36, 27 December 2006 (UTC)
What was a man able to withstand of wounds and injuries during the past (medieval times) - PART 2
Moved to combine with original section above. Anchoress 21:05, 27 December 2006 (UTC)
Trans fat question
Many food labels claim a food product has 0% trans fat, but if the first ingredient in the product is partially hydrogenated oil, how can this be so? Can the trans fat be separated out and removed? Arilcv 20:35, 27 December 2006 (UTC)
- The regulations in the United States, for example, allow less than 0.5g of trans fat per serving to be labelled as 0g. There are oil factionation methods that would probably allow the trans fat to be removed, but they may be more expensive than just using a different kind of oil (i.e. one that isn't hydrogenated). If partially hydrogenated oil is in the ingredients list, the product contains trans fat. Frankg 20:52, 27 December 2006 (UTC)
- That loophole really irritates me. Half a gram of trans fat is not necessarily trivial, especially when the serving size is like two crackers and realistically I'm going to eat a few dozen. The upshot is that "0 g trans fat" is next to meaningless, and all the good products (i.e. Smucker's Natural peanut butter) say something like "no hydrogenated oil" anyway. —Keenan Pepper 00:23, 28 December 2006 (UTC)
- I agree, this allowing companies to round down to the nearest gram is unacceptable. However, as some trans fats do occur naturally, it may be impossible to ever say a product really contains no trans fats. Perhaps what we need is an additional decimal point. Then, they could still round down, but only if the product contained less than 0.05 gram, not 0.5 gram. They will just have to decrease the serving size to a fifth of a cracker to fool us, LOL. StuRat 01:39, 28 December 2006 (UTC)
- Interesting. But I bet they wouldn't sell well if they rounded up. --Proficient 12:49, 28 December 2006 (UTC)
instrument for measuring an orifice diameter
What instrument can you use to measure a very small orifice diameter?
- How small are we talking?
- If very small maybe a travelling microscope might do the job.--Light current 21:19, 27 December 2006 (UTC)
- I was hoping to get a more quantitative measurement, to the tenth decimal. How would I use a microscope to do this?
- A very fine vernier caliper might be better. -GTBacchus 21:25, 27 December 2006 (UTC)
- Umm - "tenth decimal" - you want to measure the diameter with a precision of 10 metres i.e. 100 picometres or better ? That's the same order of magnitude as atomic bond lengths. I'm not sure that degree of precision is either realistic or meaningful. Gandalf61 22:33, 27 December 2006 (UTC)
- Choose one of:
- Calibrated wires of various sizes; see which just clears the orifice
- Lay the orifice on a calibrated reticle and use an ordinary microscope
- An optical comparator (a specialised microscope that projects onto a large screen and is used to make precision measurements of tiny things; 'shame there's no article about these, but see )
- A scanning electron microscope.
December 28
adhesives
I am looking for an adhesive that can be used with the same plastic overhead transparencies are made of but that is not water based and therefore will not oxidize a polished or anodized aluminum plate to which it is attached. I've tried petroleum based spray adhesive that works like contact cement (and probably is the spray version) but even though it is advertised as remaining clear it turns the transparency white. I need to be able to see the aluminum through the transparency. All water based adhesives either do not stick well enough or oxidize the aluminum and turn it gray or black in splotches depending on thickness. If it is possible to make one-of decals and the adhesive they use will not turn white or oxidize the aluminum plate and their cost is compariable then I interested in knowing about them as well. Thanks in advance. 71.100.6.152 01:04, 28 December 2006 (UTC)
- Cyanoacrylates ("Superglue")? FYI, transparencies are usually polyester ("Mylar").
- I've used both superglue and hot melt glue but unfortunately it only works on the edges. With the hotmelt glue I also tried application of a shrink wrap heater but the tranparency curled at the temperature of the glue. I need the adhesive to coat the entire interface between the aluminum plate and the transparency which is simply not possible (except perhaps in some sophisticated factory setup) using either one. (8.5 x 11 inch sheet) 71.100.6.152 01:31, 28 December 2006 (UTC)
Transformation of energy
Suppose a car crashes into another object. Part of the kinetic energy of the car is converted to heat and sound, and some is absorbed by the other object. Some of the energy is used in deforming parts of the car. If energy can't be destroyed, and energy was put into deforming the car, in what state does the said energy now exist? BenC7 01:35, 28 December 2006 (UTC)
- Heat. The process of deforming the car generates heat due to friction inside the material. For example, if you bend a small metal tube or stock back and forth a few times, you will feel the heat being generated because of the friction. --70.6.95.38 01:44, 28 December 2006 (UTC)
- Yeah, this is what I suspected. Ta. BenC7 04:32, 28 December 2006 (UTC)
- Some of the energy may have been converted to potential energy, like in a compressed spring that is kept from expanding by its surroundings, and may yet be harvestable (in theory). --Lambiam 08:58, 28 December 2006 (UTC)
- An obvious example of this is if the car ends up perched high up on a guard rail, retaining wall, or hillside; clearly, some portion of the vehicles' kinetic energy was expended lifting the car that ends up perched and it's now available as potential energy.
Electronics: IC that will take a 0-5V analog input and output a 0-100% PWM
PWM signals are most often generated with a microcontroller. Can you generate such a signal with just one integrated circuit (not a microcontroller). It is possible to generate such a signal with two 555 timers but I am not interested in this solution.
The output frequency should be at least 30 kHz.
- You wont get 0 to 100%. maybe 10% to 90%. Look here --Light current 01:55, 28 December 2006 (UTC)
- PWM generator chips are ICs which convert a DC level into a PWM output. Many of these are designed for use in switch mode power supplies. Unfortunately, the devices designed for switch mode power supplies tend not to allow the mark-space ratio to alter over the entire 0 - 100% range. many limit the maximum to 90% which is effectively limiting the power you can send to the motors. Devices designed as pulse generators should allow the whole range to be used. Examples are:
ST
SG1524 SMPS May operate at up to 100% duty cycle
SG3525A
Maxim
MAX038 Signal generation PWM output only between 15% and 85%. Generates triangle & sine waves too.
Atmel
U2352B PWM Generator for speed control of portable tools Includes integrated current limiting circuitry for output MOSFETs.
TI
TL494 SMPS Max 90% duty cycle
TI
UC2638 PWM generator for motor control Provides many other features for DC motor speed control.
71.100.6.152 02:00, 28 December 2006 (UTC)
- (After edit conflict) This page of PWM generators, linked from our article about PWM (and copied & pasted by 71.100.6.152), suggests that there are ICs that can do what you're after, and gives a circuit using an STMicroelectronics SG3525A chip. -- AJR | Talk 02:20, 28 December 2006 (UTC)
Ear infection
Where do ear infections come from? My doctor tells me it isn't something you catch from another person, so where does it come from? --Auximines 11:44, 28 December 2006 (UTC)
- See our articles on Otitis. --Lambiam 12:54, 28 December 2006 (UTC)
- (edit conflict) When you have any sort of congestion, the ever-present bacteria in your respiratory system tends to get pushed up your eustachian tubes into your middle ear where it finds a nice, warm, moist, very-hospitable environment and it then floureshes, producing otitis media.
I've gotten them from swimming pools (which apparently weren't adequately chlorinated). StuRat 16:01, 28 December 2006 (UTC)
- Yes, Otitis externa (a.k.a. swimmer's ear) is the other popular manifestation.
Birds' hearing
How do birds have such acute hearing when they have no external ears? Their ear is just a small hole and in most birds, it's completely covered by feathers. --84.64.4.91 11:55, 28 December 2006 (UTC)
- Apart from the owls, most birds do not have particularly good hearing, and in most cases (apart from the songbirds) their hearing is tuned to a relatively narrow waveband. Indeed, some small birds, such as the sparrow, cannot hear a low human conversation. However, all birds make up for this with excellent eyesight, and (again apart from the owls) a wide field of view.--Shantavira 14:25, 28 December 2006 (UTC)
Futuristic Glass Touch Sensor
I want to embed glass marbles in a metal casing, and give these marbles a glow from light within the casing. I need these marbles to act as switches. Microswitches behind the marbles wont do, because it will create an entry into the metal case for rain etc. when the switches are depressed, plus the simple weight of the glass marble on the switch will likely be enough to trigger it. I really want it so you just have to touch the glass and you activate that switch - would it be possible using a photo-sensitive device pressed against the other side of the marble? But since the marble is backlighted, the effect of putting your hand over marble would be to increase reflected light in dark conditions and reduce entering light in light conditions? --Username132 (talk) 13:24, 28 December 2006 (UTC)
- Using LEDs, you can very rapidly pulse (modulate) the "glowlight". This would then allow you to use a sensor that detects any rapid change in the amount of modulated glowlight reflected back to your sensor. Unless very matte-black-gloved, a finger pressed against the marble would increase the amount of reflected glowlight. And since the glowlight is pulsing, you can use electronics to distinguish the reflected glowlight from the overall ambient light.
- You can probably optimize this by clever placement of the emitters and sensor(s).
Super Duper Bright LEDs
According to Misplaced Pages's article on Candela, a 100W incandencent bulb produed about 120 000 mcd of light. On this basis, must this ebay seller be lying about his item? LED's that produce as much output as a 100W incandecent? And using just 0.1W, no less! According to Misplaced Pages on LEDs, efficiency is at about 32 lumens per watt for LEDs but for this 130 000 mcd light, that's over 17 000 lumens or 170 000 lumens per watt? --Username132 (talk) 14:17, 28 December 2006 (UTC)
- And please keep in mind that the maximum theoretical efficiency of a light source is 683 lumens/watt for a monochromatic green source at 555 nm; see Luminosity function. NO WHITE LIGHT SOURCE can approach this value because your eyes become less and less sensitive as you move away from your eyes' peak sensitivity at 555 nm.
- So they really are lying? That's unethical! --Username132 (talk) 17:57, 28 December 2006 (UTC)
- I'm afraid that's eBay for you. It is all based on trust. I once bought some binoculars on eBay and they were way below spec. And there are usually a few air guitars being "sold" on eBay too.--Shantavira 19:00, 28 December 2006 (UTC)
- Whoa! I've been looking for one of those. X (DESK|How's my driving?) 20:09, 28 December 2006 (UTC)
- I'm afraid that's eBay for you. It is all based on trust. I once bought some binoculars on eBay and they were way below spec. And there are usually a few air guitars being "sold" on eBay too.--Shantavira 19:00, 28 December 2006 (UTC)
Carbon nanotube

If super capacitors are made of nothing more than Carbon nanotubes emersed in sulphuric acid then are they used to make plates and how are the plates connected to the exterior as well as arranged inside an enclosure? Adaptron 16:18, 28 December 2006 (UTC)
- Our supercapacitor article describes them as also being made with organic aerogels which are then reduced down to extremely-porous carbon structures. Certainly supercaps were in production before the big boom in carbon nanotubes, buckyballs, and et al.
Suicidal animals
Appart from my pet turtle who "jumped" form the 5th floor window, do we know of a tendency in some individual animals to being suicidal? Keria 16:40, 28 December 2006 (UTC)
- Lemmings are the classical example, whether true or not. Members of certain political parties are also alleged to fill the bill from time-to-time.
- Lemmings are real animals? Where have I been? --Russoc4 17:27, 28 December 2006 (UTC)
- I don't believe lemmings are suicidal unless they're being chased off a cliff by Walt Disney's crew. Vranak 17:30, 28 December 2006 (UTC)
- Your turtle (like my friend's rabbit) probably wasn't suicidal. Turtles are very near sighted (in order to focus on the food directly in front of them) and so would not be able to see or understand the distance represented by more than a couple feet. At the same time, they evolved in an environment largely without cliffs and high places, and so probably lack the instinct to know that fall == bad. So, I am guessing it was more a case of the turtle being too blind and dumb to know better. Dragons flight 17:36, 28 December 2006 (UTC)
- Whales beaching themselves is one possibility. Other explanations have been offered, but the way they frequently beach themselves immediately again once pushed out to sea makes me rather suspicious. StuRat 19:20, 28 December 2006 (UTC)
- Animals can't be suicidal, unless they've already passed on their genes for sure, or can think complex enough to be able to do it. How is the validity of that statement? X (DESK|How's my driving?) 20:07, 28 December 2006 (UTC)
- I think you're saying that their actions don't count as suicide unless they understand what death is. I think some animals, besides people, do have a basic understanding of death. Elephants, for example, go to the "elephant graveyard" and visit the bones of their relatives periodically. They appear to have an emotional reaction. StuRat 20:58, 28 December 2006 (UTC)
wearing rubber gloves
I've noticed that some restaurant workers put on latex glove to handle food but then touch everything from garbage to scratching their private parts with the gloves on as if the true purpose for the gloves is to keep their own flesh from becoming contaminated. Is this lack of sufficient regulation or training or just failure to comply on the part of such workers. (BTW Even emergency room intake personnel have been seen doing the same thing. Hope it is not true for nurses and doctors.) -- 71.100.6.152
- You're right, this is a common practice. There need to be procedures set up to avoid this. For example, at the Subway sandwich shop, employees remove and toss out their disposable gloves when they handle money, then put a new set on for the next customer. In larger restaurants the best way to handle it would be to have one person who handles the register and another who handles the food. Garbage and cleaning tables should also be handled by somebody else. Cooked and uncooked foods should also be handled by different people. At hospitals, failure to establish and follow proper hygiene procedures regularly causes infections and deaths. The root problem is insufficient punishment for those who violate procedures. For example, any employee caught not washing their hands after using the toilet should be fired immediately. Unfortunately, that almost never happens. StuRat 19:14, 28 December 2006 (UTC)
- Yes I think the Subway method is quite good. I go there regularly and have never seen any unhygenic manoevers. On thing that bothers me tho' is when they put on the platic gloves, they actually touch them with ungloved hands 8-)--Light current 19:20, 28 December 2006 (UTC)
- Good point, they need a fixture that holds the sterile gloves in place while they put them on. StuRat 19:56, 28 December 2006 (UTC)
- They better not touch the fixture, or I'd be worried! X (DESK|How's my driving?) 20:04, 28 December 2006 (UTC)
- Pfft, I worked in a subway (eeeat fresh!) and you don't want to know what happened to the food before it entered that plastic bin and made it's way to the front. Gloves are there to placate germophobes. If you want sterile food, simply raise it's entire mass to 175F, cool, and enjoy! As for me, what's a few more germs? As long as I don't get a booger shot on my sandwich, I won't be grossed out in the least. --Anonymous
- Another good point, the entire food prep area should be visible to the public, not just the final sandwich prep area, if we are to shame them into proper hygiene procedures. StuRat 20:49, 28 December 2006 (UTC)
Why does chalk cause fever?
Some kids have used (or still use) as school-evasion strategies chalk, because chewing it caused fever for a short time, without causing any noticeable side-effect. What might be the cause of this phenomena? What effect does a bit of chalk have on the organism? Why? Is it even dangerous? Don't consider this question as a seeking of medical advice... I was just wondering... --86.125.180.178 18:06, 28 December 2006 (UTC)
- Interesting question and I don't know the full extent of the answer. Naturally occurring chalk is a form of Limestone (calcium carbonate) that is very soft and porous. However, regular chalkboard chalk that is commonly used in schools is made from the mineral Gypsum (calcium sulfate) rather than calcium carbonate. I do know that many people, including myself, are mildly allergic to the calcium sulfate found in processed chalk, it is known to cause respiratory and breathing problems in many people but I have never heard of it causing a fever. Perhaps this is just another symptom of an allergic reaction that some people experience? I would be interested to hear any other insights--Nebular110 18:19, 28 December 2006 (UTC)
- I don't know either, but calcium sulfate (aka E516) is commonly found in bread (such as baker's delight bread) as a "Flour treatment agent". —Pengo 01:01, 29 December 2006 (UTC)
This is ridiculous. If someone can supply enough detail to make a brief trial, I guarantee we can get this published and it will be in every newspaper in the country and twice around the world within a week. This would be an extremely well-known phenomenon if it were true. Many medical treatments in the late 19th and early 20th centuries for a variety of infections involved intentionally attempting to induce a fever. Dangerous infections were even caused intentionally to try to cure worse ones. If a fever could be induced by something as simple as calcium sulfate or calcium carbonate every doctor would know it and it would be on every differential diagnosis list as a cause of FUO. Thanks for the chuckle. alteripse 02:34, 29 December 2006 (UTC)
Soret band? yes/no
Does vitamin B12 emit light in the visible range like a true heme, or does it lack a strong absorption band?--74.66.242.190 18:10, 28 December 2006 (UTC)
Painful but nonlethal stabbing
What places on the human body can be stabbed, without killing, but so painful the person stabbed is forced to surrender? PitchBlack 18:18, 28 December 2006 (UTC)
- The places with the most nerve endings and the least arteries. e.g. feet and hands. Why do you ask?--Shantavira 19:07, 28 December 2006 (UTC)
- The joints are particularly fragile and vulnerable, and damage to them will severely disable someone. I'm not so sure about the chances of killing someone with a joint injury, but I don't hear about that a lot. — Kieff 21:51, 28 December 2006 (UTC)
If you can get a clean stab into someone, chances are that you could disable them without resorting to such extreme measures in the first place. Vranak
Suffering from exposure to Frost, cold and water
If a guy falls into a lake out the wild in forexample harsh winter weather with -15 C degrees (nevermind the ice, lets just say it cracks somehow) and his nearby friend manages to reach him and save him by grabbing him with one arm (a quite strong arm mind you) then i guess the poor bastard who fell into the water is quite done for. Any idea/opinions on how fast he would freeze to death ?
And quite interesting i think, how would this friend/guy who pulled him up fare ? how would his arm and hand fare ? (Ofc, his hand has to be put into the icy water and get wet to pull his friend up - say he's quite strong and able to do so with only one arm) I take it chances would be BIG for him to soon suffer from frostbite, or would he DEFINATELY suffer from it without a shadow of a doubt in such a harsh climate? At least his fingers and hand would be greatly in risk, no ? However, if he quickly dry the arm and hand as best he can with something dry and then wraps arm/hand/fingers in as much cloth he can to generate warmth, he should be able to fully "recover" and save his arm/hand/fingers, no ? - even though they are out in the wild with extreme cold...
The one who fell into the water would probably go into shock or something and he would freeze to death (in minutes, wouldn't you agree?)
The one who 'saves' him and gets his arm wet tho, he has quite realistic chances of saving his arm if he quickly does as i described, no ?
By the way : is there any page(s) you could link me to that tells of 'freezing to death' and the process as it unfolds until ones death?
and the process of suffering from exposure to cold and frost in any way ?
I don't know what to search for then.... i have searched for Frostbite ofc, but it doesnt fill all my needs for information on the subject.
Thank you, Krikkert
- Well, the water, being liquid, can't be colder than about 0C (you said it was a lake, so no salt water here) . So frostbite merely from contact with the water isn't an issue. The issue is how quickly one's body loses heat into the chilly water. I can assure you that it is possible to survive a dunk into chilly water; the key is how long you remain in the water and how much additional heat you lose once removed from the water. Your body has quite a bit of thermal mass and your fat layer provides some thermal insulation, enough to withstand at least a few minutes . I suppose being rescued into -15C air is probably a pretty bad situation, but even then it depends on things like wind chill effect and whether there's any shelter nearby.
On the sun...
I was reading the article on the sun, and was absolutely intrigued by this line- 'Tidal effects from the planets do not significantly affect the shape of the Sun, although the Sun itself orbits the center of mass of the solar system, which is located nearly a solar radius away from the center of the Sun mostly because of the large mass of Jupiter.' Now, this raised a number of questions to me-
- Where exactly is this center of orbit? 'Nearly a solar radius away from the center of the Sun' would mean within the sun itself, would it not?
- Does this orbit change? Surely, at different points in the orbits of the larger planets, this center of gravity for the solar system would be at different points?
- How long does the sun take to orbit this?
- Do the planets orbit the sun, as I have always been taught, or do they orbit this center of gravity?
- Does the sun move at all, apart from this?
- What about with other bodies? Surely, if this is happening to the sun, it could also happen to the earth, so that it circles a point slightly outside its own central point, because of the center point of gravity within the Earth-Moon system?
I understand astrophysics as far as getting me an A* at GCSE science goes, but not far beyond that. Any answers to these questions would be very appreciated. Thanks! J Milburn 20:19, 28 December 2006 (UTC)
- The center of rotation of a system of two objects is called a barycenter. The only barycenter well outside the larger of the two objects I know of in our solar system is Pluto, due to it's large moon Charon. (Check out the barycenter link, it has many of the answers you seek.) StuRat 20:27, 28 December 2006 (UTC)
- It may also help to remember that if you exclude the sun, Jupiter makes up more then half the mass of the solar system, this means if you combined all the other planets you still won't have as much mass as Jupiter! So the other planets may have a small effect on the sun, but not nearly as much as Jupiter. Also, when you say, is it orbiting the sun or the centre of gravity those are not really mutually exclusive. Like in any gravity system, even the earth/you system, two bodies exert force on each other. Pound for pound, you exert as much gravitational force on the earth as it does on you. If you were in orbit it would seem that you were spinning around the earth and the earth was stationary, now imagine you get bigger and bigger until you have as much mass as the earth, all of a sudden, the earth is orbiting you as much as you are orbiting the earth! And to the outside observer it looks like you are actually both orbiting some invisible point in-between you. Vespine 21:39, 28 December 2006 (UTC)
The barycenter article was very helpful, the animated gifs basically explained the whole thing to me! When combined with the description given by Vespine, I basically have the answers I was looking for. Thanks! One of my other questions was not really related to the others, however- is the sun moving through space? Perhaps orbiting something big, perhaps aimlesly floating, perhaps being thrown outwards? J Milburn 22:51, 28 December 2006 (UTC)
- The sun as the centre of our solar system is in orbit around the galactic centre of our galaxy the Milky way. It's ALL orbits man!:) Vespine 23:02, 28 December 2006 (UTC)
Is there really any difference between "friction" and "collision"
Is this a meaningless or illusory distinction? Is it meaningful only in the context of classical mechanics? NoClutter 20:38, 28 December 2006 (UTC)
- You could read the Friction and Collision pages and read the definitions to see why they are different. :-) Imaninjapirate 01:22, 29 December 2006 (UTC)
Faster-than-light aspects
May be I'm missing the relevant points that already exist, but is it theoretically possible that if the faster-than-light speed is achieved, the spaceship pilot will see only darkness, and also because the spaceship moves faster than light, it will be invisible in all spectra? (if so, I assume that the spaceship would be virtually invisible for all unless the pilot reduces the speed). Is it also possible that the FTL would be unachievable in the gravity fields due to indescribable gees? --Brand спойт 20:45, 28 December 2006 (UTC)
- No, no, and sorry but no. Theoratically FTL is not possible, c IS the fastest absolutely anything can go. Maybe some reading of the Speed of light article may help. People can make guesses as to what could happen if FTL was possible, but those guesses would go against currently held theories. Vespine 21:27, 28 December 2006 (UTC)
- Theoretically, FTL is possible. See Tachyon (which in not a Star Trek invention). What you can't do (as mere matter) is reach the speed of light, by either accelerating, or in the case of tachyons, decelerating. (On the other hand, theoratically, StuRat is not possible.) Clarityfiend 01:22, 29 December 2006 (UTC)
- But to REALLY stretch the imagination, I'll let go my previous bias to reality... A pilot travelling faster then light would not just see darkness, just as a pilot flying faster then sound doesn't go deaf. A pilot exposed to faster then sound airflow would probably go deaf, if his head wasn't torn off by the wind first, because the air pressure would exceed the speed of sound (sic).. So, a pilot going FTL would probably see BRIGHT WHITE rather then dark because the light pressure exceeds the speed of light... NEXT: Moving faster then the speed of sound creates a sonic BOOM, far from silent running. So, similarly, something travelling FTL might create a lux boom, where light stacks on top of itself and reaches an observer all at once. or, if say the FTL traveller was in orbit, they would appear as a SOLID ring in all places of the orbit at once! And finally, gees are only felt during acceleration, so no, that wouldn't be a problem as long as you didn't accelerate too fast. The shuttle accelerates at 3g, or about 30m/s per second, at that rate, you could reach the speed of light in about 115 days, according to my calculations... Can someone check that? That seems low, c doesn't seem so infinite when you put it like that:)Vespine 22:59, 28 December 2006 (UTC)
- Yes, 115 days is about right. Of course, staying at 3g for 115 days would be very bad for your health. It takes about a year at 1g, which would be far more pleasant. These calcs, of course, use Newtonian physics; relativity would become more important closer to the speed of light. Specifically, the amount of elapsed time would appear to be less to an observer aboard the ship, by about half, I would guess. StuRat 01:00, 29 December 2006 (UTC)
- You'd never get to the speed of light that way, according to special relativity. Just closer and closer and closer. (I have actually calculated speed as a function of time at "constant acceleration" in special relativity, for what it's worth—which, of course, isn't much.) -- SCZenz 01:12, 29 December 2006 (UTC)
stream velocities for Red River (USA)
Hi, I'm trying to find stream velocity for various points on the Red River (USA). I mean the Red River that forms the border between Oklahoma and Texas, then Arkansas and Texas, then entering Louisana and joining the Mississipii.
I'd like to find a map of the avg velocites for different months, but I haven't been able to find anything at all. thanks in advance68.221.112.103 22:14, 28 December 2006 (UTC)
- Do you mean flow rate rather than velocity? Historical (and realtime) stream gauge data can be found at the USGS National Water Information System web site.EricR 23:06, 28 December 2006 (UTC)
No, I really mean velocity--I'm planning a rafting trip. I saw that site and tried to use it, but never got it to give me the data:it assumes I know the site names in its database a priori.
thanks for checking on that. any other ideas, even offline?
68.221.112.103 23:15, 28 December 2006 (UTC)
Egg shells
What is the best way to either dissolve or crush (Finely powered) Egg shells for the purpose of human consumption? 71.100.6.152 22:55, 28 December 2006 (UTC)
December 29
Relativity of light
I was reading about the Theory of Special Relativity in The Elegant Universe, and Greene said that if you chase a beam of light at light speed, the beam of light will continue to get farther away from you at the speed of light. This made my head hurt. Is it just one of those strange quirks of physics that you just have accept because there is proof for it, no matter how weird it is; or is there a real qualitative reason behind this phenomenon? Interesting stuff, this special and general relativity. :-) Imaninjapirate 01:33, 29 December 2006 (UTC)
- The fact that the speed of light is exactly the same in all inertial reference frames is an axiom (that is, an assumption) of the theory of relativity. It does not seem to have been intuitively obvious to anyone except Einstein himself, but it does turn out to result in theories that are correct to very high precision. -- SCZenz 01:55, 29 December 2006 (UTC)