| Revision as of 12:13, 8 July 2006 view sourceTevildo (talk | contribs)Extended confirmed users18,696 editsm Minor typos/gramattical issues fixed← Previous edit | Revision as of 06:34, 10 July 2006 view source YurikBot (talk | contribs)278,165 editsm robot Adding: simple:Atomic theoryNext edit → | ||
| Line 78: | Line 78: | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Revision as of 06:34, 10 July 2006
In chemistry and physics, atomic theory is a theory of the nature of matter. It states that all matter is composed of atoms. The philosophical background of the atomic theory is called atomism. The theory applies to the common phases of matter, namely solids, liquids and gases, as directly experienced on Earth. Strictly speaking, it is not the appropriate theory for plasmas or neutron stars where unusual environments such as extremes of temperature or density prevent atoms from forming.
Importance
Arguably, the atomic theory is one of the most important theories in the history of science, with wide-ranging implications for both pure and applied science. The theory is largely credited to John Dalton, an 18th- and 19th century British chemist and physicist.
Modern chemistry (and biochemistry) is based upon the theory that all matter is made up of atoms of different elements, which cannot be transmuted by chemical means. In turn, chemistry has allowed for the development of the pharmaceutical industry, the petrochemical industry, and many others.
Much of thermodynamics is understandable in terms of kinetic theory, whereby gases are considered to be made up of either atoms or molecules, behaving in accordance with Newton's laws of motion. This was, in turn, a large driving force behind the industrial revolution.
Indeed, many macroscopic properties of matter are best understood in terms of atoms. Other examples include friction, material science and semiconductor theory. The last-named is particularly important, as it is the foundation of electronics.
Historical precursors
Early atomism
Main article: AtomismFrom the 6th century BC, Hindu, Buddhist and Jaina philosophers in ancient India developed the earliest atomic theories. The first philosopher who formulated ideas about the atom in a systematic manner was Kanada who lived in the 6th century BC. Another Indian philosopher, Pakudha Katyayana, who also lived in the 6th century BC and was a contemporary of Gautama Buddha, had also propounded ideas about the atomic constitution of the material world. Indian atomists believed that an atom could be one of up to six elements, with each element having up to 24 properties. They developed detailed theories of how atoms could combine, react, vibrate, move, and perform other actions, and had particularly elaborate theories of how atoms combine, which explained how atoms first combine in pairs, and then group into trios of pairs, which are the smallest visible units of matter. This parallels with the structure of modern atomic theory, in which pairs or triplets of supposedly fundamental quarks combine to create most typical forms of matter. They had also suggested the possibility of splitting an atom which, as we know today, is the source of atomic energy. (See Indian atomism for more details.)
Democritus and Leucippus, Greek philosophers in the 5th century BC, presented a theory of atoms. (See Atomism for more details.) The Greeks believed that atoms were all made of the same material but had different shapes and sizes, which determined the physical properties of the material. For instance, the atoms of a liquid were thought to be smooth, allowing them to slide over each other.
During the Middle Ages (the Islamic Golden Age), Islamic atomists developed atomic theories that represent a synthesis of both Greek and Indian atomism. (See Islamic atomism for more details.) Older Greek and Indian ideas were further developed by Islamic atomists, along with new Islamic ideas, such as the possibility of there being particles smaller than an atom. As Islamic influence began spreading through Europe, the ideas of Islamic atomism, along with the older ideas of Greek and Indian atomism, spread throughout Europe by the end of the Middle Ages.
It should be noted that these theories were purely philosophical, and not founded in scientific experimentation.
Birth of modern atomic theory
In 1808, John Dalton proposed that an element is composed of atoms of a single, unique type, and that although their shape and structure was immutable, atoms of different elements could combine to form more complex structures (chemical compounds). He proposed this theory to explain the law of multiple proportions — that is, if two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.
One such combination he is believed to have studied involves nitrous oxide (NO) and oxygen (O2). In one combination, these gases formed dinitrogen trioxide (N2O3), but when he repeated the combination with double the amount of oxygen (a ratio of 1:2), they instead formed nitrogen dioxide (NO2).
4NO + O2 → 2N2O3
4NO + 2O2 → 4NO2
In 1827, biologist Robert Brown observed that pollen grains floating in water constantly jiggled about for no apparent reason. In 1905, Albert Einstein theorised that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a mathematical theory around it. This theory was validated experimentally in 1911 by French physicist Jean Perrin, thus providing additional validation for atomic theory.
Discovery of subatomic particles
For much of this time, atoms were thought to be the smallest possible division of matter. However, in 1897, J.J. Thomson published his work proving that cathode rays are made of negatively charged particles (electrons). Since cathode rays are emitted from matter, this proved that atoms are made up of subatomic particles and are therefore divisible, and not the indivisible atomos postulated by Democritus. Physicists later invented a new term for such indivisible units, "elementary particles", since the word atom had come into its common modern use.
Study of atomic structure
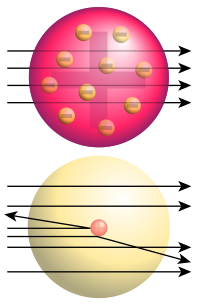
Top: Expected results: alpha particles passing through the plum pudding model of the atom undisturbed.
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge.
At first, it was believed that the light electrons were distributed more or less uniformily in a sea of positive charge (the plum pudding model). However, an experiment conducted in 1909 by colleagues of Ernest Rutherford demonstrated that atoms have most of their mass and positive charge concentrated in a very small fraction of their volume, a region which Rutherford assumed to be at the very center of the atom. In the gold foil experiment, alpha particles (emitted by polonium) were shot through a sheet of gold (striking a fluorescent screen on the other side). The experimenters expected all the alpha particles to pass through without significant deflection, given the uniform distribution of positive charge in the plum pudding model. On the contrary, about 1 in 8000 of the alpha particles were heavily deflected (by more than 90 degrees). This led Rutherford to propose the planetary model of the atom in which pointlike electrons orbited in the space around a massive compact nucleus like planets orbiting the Sun.
Ten years later, Rutherford discovered that he could transmute one element into another by bombarding it with alpha particles. In each of these cases, hydrogen nuclei were emitted. By comparing nuclear masses to charges he found that the positive charge of any atom could be equated to that of an integer number of hydrogen nuclei. Rutherford had discovered the proton. Further experimentation by Rutherford found that the nuclear mass of most atoms exceeded that of the protons it possessed; this led him to postulate the existence of neutrons, whose existence would be proven in 1932 by James Chadwick.
The planetary model of the atom still had shortcomings. Firstly, a moving electric charge emits electromagnetic waves; according to classical electromagnetism, an orbiting charge would steadily lose energy and spiral towards the nucleus, colliding with it in a tiny fraction of a second. Another phenomenon the model did not explain was why excited atoms only emit light with certain discrete spectra.
Quantum theory revolutionized physics at the beginning of the 20th century when Max Planck and Albert Einstein postulated that light energy is emitted or absorbed in fixed amounts known as quanta. In 1913, Niels Bohr used this idea in his Bohr model of the atom, in which the electrons could only orbit the nucleus in particular circular orbits with fixed angular momentum and energy. They were not allowed to spiral into the nucleus, because they could not lose energy in a continuous manner; they could only make quantum leaps between fixed energy levels. Bohr's model was extended by Arnold Sommerfeld in 1916 to include elliptical orbits, using a quantization of generalized momentum.
The ad hoc Bohr-Sommerfeld model was extremely difficult to use, but it made impressive predictions in agreement with certain spectral properties. However, the model was unable to explain multielectron atoms, predict transition rates or describe fine and hyperfine structure. In 1925, Erwin Schrödinger developed a full theory of quantum mechanics, described by the Schrödinger equation. Together with Wolfgang Pauli's exclusion principle, this allowed the study of atoms with great precision when digital computers became available. Even today, these theories are used in the Hartree-Fock quantum chemical method to determine the energy levels of atoms. Further refinements of quantum theory such as the Dirac equation and quantum field theory made smaller impacts on the theory of atoms.
Another model of historical interest, proposed by Gilbert N. Lewis in 1916, had cubical atoms with electrons statically held at the corners. The cubes could share edges or faces to form chemical bonds. This model was created to account for chemical phenomena such as bonding, rather than physical phenomena such as atomic spectra.
See also
- History of thermodynamics
- Kinetic theory
- Development of Quantum Theory
- Quantum Chemistry
- John Dalton
Related lists
- Timeline of chemical element discovery
- Timeline of quantum mechanics, molecular physics, atomic physics, nuclear physics, and particle physics
- Timeline of thermodynamics, statistical mechanics, and random processes
References
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "History of atomic theory" – news · newspapers · books · scholar · JSTOR (Learn how and when to remove this message) |