This is an old revision of this page, as edited by Doc James (talk | contribs) at 00:57, 27 December 2010 (moved from Causes_of_schizophrenia). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 00:57, 27 December 2010 by Doc James (talk | contribs) (moved from Causes_of_schizophrenia)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)Structural
Studies have tended to show various subtle average differences in the volume of certain areas of brain structure between people with and without diagnoses of schizophrenia, although it has become increasingly clear that there is no single pathological neuropsychological or structural neuroanatomic profile, due partly to heterogeneity within the disorder. The most consistent volumetric findings are (first-onset patient vs control group averages), slightly less grey matter volume and slightly increased ventricular volume in certain areas of the brain. The two findings are thought to be linked. Although the differences are found in first-episode cases, grey matter volumes are partly a result of life experiences, drugs and malnutrition etc., so the exact role in the disorder is unclear. In addition, ventricle volumes are amongst the mostly highly variable and environmentally influenced aspects of brain structure, and the percentage difference in group averages in schizophrenia studies has been described as "not a very profound difference in the context of normal variation." A slightly smaller than average whole-brain volume has also been also been found, and slightly smaller hippocampal volume in terms of group averages. These differences may be present from birth or develop later, and there is substantial variation between individuals.
Most schizophrenia studies have found average reduced volume of the left medial temporal lobe and left superior temporal gyrus, and half of studies have revealed deficits in certain areas of the frontal gyrus, parahippocampal gyrus and temporal gyrus. However, at variance with some findings in individuals with chronic schizophrenia (where use of antipsychotics and other factors may have a confounding effect), significant group differences of temporal lobe and amygdala volumes are not shown in first-episode patients on average. The neurobiological abnormalities are so varied that no single abnormality is observed across the entire group of people with DSM-IV–defined schizophrenia. In addition, it remains unclear whether the structural differences are unique to schizophrenia or cut across the traditional diagnostic boundaries between schizophrenia and affective disorders - though perhaps being unique to conditions with psychotic features.
Studies of the rare childhood-onset schizophrenia (before age 13) indicate a greater-than-normal loss of grey matter over several years, progressing from the back of the brain to the front, levelling out in early adulthood. Such a pattern of "pruning" occurs as part of normal brain development but appears to be exaggerated in childhood-onset psychotic diagnoses, particularly schizophrenia. Abnormalities in the volume of the ventricles or frontal lobes have also been found in several studies but not in others. Volume changes are most likely glial and vascular rather than purely neuronal, and reduction in grey matter may primarily reflect a reduction of neuropil rather than a deficit in the total number of neurons. Other studies, especially some computational studies, have shown that a reduction in the number of neurons can cause psychotic symptoms. Studies to date have been based on small numbers of the most severe and treatment-resistant patients taking antipsychotics.
Functional
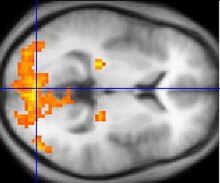
Some studies using neuropsychological tests and brain imaging technologies such as fMRI and PET to examine functional differences in brain activity have shown that differences seem to most commonly occur in the frontal lobes, hippocampus, and temporal lobes. Abnormalities of the kind shown are linked to the same neurocognitive deficits often associated with schizophrenia, particularly in areas of memory, attention, problem solving, executive function, and social cognition. Observations of the frontal lobe in patients with schizophrenia are inconsistent: While many studies have found abnormalities, others have found no or only a statistically insignificant difference. Data from a PET study suggests that the less the frontal lobes are activated during a working memory task, the greater the increase in abnormal dopamine activity in the striatum, thought to be related to the neurocognitive deficits in schizophrenia.
Electroencephalograph (EEG) recordings of persons with schizophrenia performing perception oriented tasks showed an absence of gamma band activity in the brain, indicating weak integration of critical neural networks in the brain. Those who experienced intense hallucinations, delusions and disorganized thinking showed the lowest frequency synchronization. None of the drugs taken by the persons scanned had moved neural synchrony back into the gamma frequency range. Gamma band and working memory alterations may be related to alterations in interneurons that produce the neurotransmitter GABA.
Atypical connectivity in the default network and other resting-state networks in the brain has been observed in schizophrenic patients. The greater connectivity in the default network and the task-positive network may reflect excessive orientation of attention to introspection and to extrospection, respectively, and the greater anti-correlation between the two networks suggests excessive rivalry between the networks. Increased deactivation of specific default-network regions is associated with the positive symptoms of schizophrenia.
Dopamine
Main article: Dopamine hypothesis of schizophrenia
Particular focus has been placed upon the function of dopamine in the mesolimbic pathway of the brain. This focus largely resulted from the accidental finding that a drug group which blocks dopamine function, known as the phenothiazines, could reduce psychotic symptoms. An influential theory, known as the "dopamine hypothesis of schizophrenia", proposed that a malfunction involving dopamine pathways was therefore the cause of (the positive symptoms of) schizophrenia. Evidence for this theory includes findings that the potency of many antipsychotics is correlated with their affinity to dopamine D2 receptors; and the exacerbatory effects of a dopamine agonist (amphetamine) and a dopamine beta hydroxylase inhibitor (disulfiram) on schizophrenia; and post-mortem studies initially suggested increased density of dopamine D2 receptors in the striatum. Such high levels of D receptors intensify brain signals in schizophrenia and causes positive symptoms such as hallucinations and paranoia. Impaired glutamate (a neurotransmitter which directs neuron to pass along an impulse) activity appears to be another source of schizophrenia symptoms.
However, there was controversy and conflicting findings over whether post-mortem findings resulted from chronic antipsychotic treatment. Compared to the success of postmortem studies in finding profound changes of dopamine receptors, imaging studies using SPET and PET methods in drug naive patients have generally failed to find any difference in dopamine D2 receptor density compared to controls. Comparable findings in longitudinal studies show: " Particular emphasis is given to methodological limitations in the existing literature, including lack of reliability data, clinical heterogeneity among studies, and inadequate study designs and statistic," suggestions are made for improving future longitudinal neuroimaging studies of treatment effects in schizophrenia A recent review of imaging studies in schizophrenia shows confidence in the techniques, while disussing such operator error. In 2007 one report said, "During the last decade, results of brain imaging studies by use of PET and SPET in schizophrenic patients showed a clear dysregulation of the dopaminergic system."
Recent findings from meta-analyses suggest that there may be a small elevation in dopamine D2 receptors in drug-free patients with schizophrenia, but the degree of overlap between patients and controls makes it unlikely that this is clinically meaningful. In addition, newer antipsychotic medication (called atypical antipsychotic medication) can be as potent as older medication (called typical antipsychotic medication) while also affecting serotonin function and having somewhat less of a dopamine blocking effect. In addition, dopamine pathway dysfunction has not been reliably shown to correlate with symptom onset or severity. HVA levels correlate trendwise to symptoms severity. During the application of debrisoquin this correlation becomes significant
Giving a more precise explanation of this discrepancy in d2 receptor radioligand imaging measurements involves the monomer and dimer ratio, Dr Philip Seeman has said: "In schizophrenia, therefore, the density of methylspiperone sites rises, reflecting an increase in monomers, while the density of raclopride sites remains the same, indicating that the total population of D2 monomers and dimers does not change.". With this difference in measurement technique in mind; the above mentioned meta analysis uses results from 10 different ligands.
It has been said that, "...Numerous postmortem studies have consistently revealed D2 receptors to be elevated in the striata of patients with schizophrenia". However, the authors were concerned the effect of medication may not have been fully accounted for. The study introduced an experiment by Abi-Dargham et al. in which it was shown medication free live schizophrenics had more d2 receptors involved in the schizophrenic process and more dopamine. Since then another study has shown such elevated percentages in d2 receptors is brain-wide (using a different ligand, which did not need dopamine depletion) In a 2009 study Annisa Abi-Dagham et al. confirmed the findings of her previous study regarding increased baseline d2 receptors in schizophrenics and showing a correlation between this magnitude and the result of amphetamine stimulation experiments.
Some animal models of psychosis are similar to those for addiction - displaying increased locomotor activity For those female animals with previous sexual experience, amphetamine stimulation happens faster than for virgins. There is no study on male equivalent because the studies are meant to explain why females experience addiction earlier than males.
Even in 1986 the effect of antipsychotics on receptor measurement was controversial. An article in Science sought to clarify whether the increase was solely due to medication by using drug naive schizophrenics: "The finding that D2 dopamine receptors are substantially increased in schizophrenic patients who have never been treated with neuroleptic drugs raises the possibility that dopamine receptors are involved in the schizophrenic disease process itself. Alternatively, the increased D2 receptor number may reflect presynaptic factors such as increased endogenous dopamine levels (16). In either case, our findings support the hypothesis that dopamine receptor abnormalities are present in untreated schizophrenic patients." (The experiment used 3-N-methylspiperone- the same as mentioned by Dr Seeman detects d2 monomers and binding was double that of controls.)
It is still thought that dopamine mesolimbic pathways may be hyperactive, resulting in hyperstimulation of D2 receptors and positive symptoms. There is also growing evidence that, conversely, mesocortical pathway dopamine projections to the prefrontal cortex might be hypoactive (underactive), resulting in hypostimulation of D1 receptors, which may be related to negative symptoms and cognitive impairment. The overactivity and underactivity in these different regions may be linked, and may not be due to a primary dysfunction of dopamine systems but to more general neurodevelopmental issues that precede them. Increased dopamine sensitivity may be a common final pathway.
Another reliable finding, repeatedly found, is that there is a some sixfold excess of binding sites insensitive to a certain testing agent (raclopride) Dr Seeman later said this increase was probably due to the increase in d2 monomers. Such an increase in monomers, occurs via the cooperativity mechanism which is responsible for d2high and d2low, the supersensitive and lowsensitivity states of the d2 dopamine receptor. More specifically, "an increase in monomers, may be one basis for dopamine supersensitivity."
Another one of Philip Seeman's findings was that the dopamine D2 receptor protein looked abnormal in schizophrenia. Proteins change states by flexing. The activating of the protein by folding could be permanent or fluctuating, just like the courses of patients' illnesses waxes and wanes. Increased folding of a protein leads to increased risk of 'additional fragments' forming The schizophrenic d2 receptor has a unique additional fragment when digested by papain in the test-tube, but none of the controls exhibited the same fragment. The D2 receptor in schizophrenia are thus in a highly active state as found by Philip Seeman et al.
Glutamate
Main article: Glutamate hypothesis of schizophreniaInterest has also focused on the neurotransmitter glutamate and the reduced function of the NMDA glutamate receptor in schizophrenia. This has largely been suggested by abnormally low levels of glutamate receptors found in postmortem brains of people previously diagnosed with schizophrenia and the discovery that the glutamate blocking drugs such as phencyclidine and ketamine can mimic the symptoms and cognitive problems associated with the condition. The fact that reduced glutamate function is linked to poor performance on tests requiring frontal lobe and hippocampal function and that glutamate can affect dopamine function, all of which have been implicated in schizophrenia, have suggested an important mediating (and possibly causal) role of glutamate pathways in schizophrenia. Further support of this theory has come from preliminary trials suggesting the efficacy of coagonists at the NMDA receptor complex in reducing some of the positive symptoms of schizophrenia.
Other
Dyregulation of neural calcium homeostasis has been hypothesized to be a link between the glutamate and dopaminergic abnormalities and some small studies have indicated that calcium channel blocking agents can lead to improvements on some measures in schizophrenia with tardive dyskinesia.
There is evidence of irregular cellular metabolism and oxidative stress in the prefrontal cortex in schizophrenia, involving increased glucose demand and/or cellular hypoxia.
Mutations in the gene for brain-derived neurotrophic factor (BDNF) have been reported to be a risk factor for the disease.
References
- Flashman LA, Green MF (2004). "Review of cognition and brain structure in schizophrenia: profiles, longitudinal course, and effects of treatment". Psychiatr. Clin. North Am. 27 (1): 1–18, vii. doi:10.1016/S0193-953X(03)00105-9. PMID 15062627.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA (2006). "Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies". Br J Psychiatry. 188: 510–8. doi:10.1192/bjp.188.6.510. PMID 16738340.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Allen JS, Damasio H, Grabowski TJ (2002). "Normal neuroanatomical variation in the human brain: an MRI-volumetric study". American journal of physical anthropology. 118 (4): 341–58. doi:10.1002/ajpa.10092. PMID 12124914.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Honea R, Crow TJ, Passingham D, Mackay CE (2005). "Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies". American Journal of Psychiatry. 162 (12): 2233–45. doi:10.1176/appi.ajp.162.12.2233. PMID 16330585.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Vita A, De Peri L, Silenzi C, Dieci M (2006). "Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies". Schizophrenia Research. 82 (1): 75–88. doi:10.1016/j.schres.2005.11.004. PMID 16377156.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Prasad KM, Keshavan MS (2008). "Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct "extended endophenotypes"?". Schizophrenia Bulletin. 34 (4): 774–90. doi:10.1093/schbul/sbn017. PMC 2632444. PMID 18408230.
{{cite journal}}: Unknown parameter|month=ignored (help) - Hoffman R, McGlashan T (2001). "Neural network models of schizophrenia". Neuroscientist. 7 (5): 441–54. doi:10.1177/107385840100700513. PMID 11597103.
{{cite journal}}: Unknown parameter|month=ignored (help) - Arango C, Moreno C, Martínez S; et al. (2008). "Longitudinal brain changes in early-onset psychosis". Schizophrenia Bulletin. 34 (2): 341–53. doi:10.1093/schbul/sbm157. PMC 2632400. PMID 18234701.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Green, Michael (2001). Schizophrenia revealed: from neurons to social interactions. New York: Norton. ISBN 0-393-70334-7.
- Park S, Holzman PS (1992). "Schizophrenics show spatial working memory deficits". Arch. Gen. Psychiatry. 49 (12): 975–82. PMID 1449384.
{{cite journal}}: Unknown parameter|month=ignored (help) - Wible CG, Shenton ME, Hokama H; et al. (1995). "Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study". Arch. Gen. Psychiatry. 52 (4): 279–88. PMID 7702444.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Selemon LD, Rajkowska G, Goldman-Rakic PS (1998). "Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method". J. Comp. Neurol. 392 (3): 402–12. doi:10.1002/(SICI)1096-9861(19980316)392:3<402::AID-CNE9>3.0.CO;2-5. PMID 9511926.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Meyer-Lindenberg A, Miletich RS, Kohn PD; et al. (2002). "Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia". Nat. Neurosci. 5 (3): 267–71. doi:10.1038/nn804. PMID 11865311.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Spencer KM, Nestor PG, Perlmutter R; et al. (2004). "Neural synchrony indexes disordered perception and cognition in schizophrenia". Proc. Natl. Acad. Sci. U.S.A. 101 (49): 17288–93. doi:10.1073/pnas.0406074101. PMC 535363. PMID 15546988.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS (2009). "Default-mode brain dysfunction in mental disorders: a systematic review". Neurosci Biobehav Rev. 33 (3): 279–96. doi:10.1016/j.neubiorev.2008.09.002. PMID 18824195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Experimental Eye Research Volume 83, Issue 5, November 2006, Pages 1246-1251 Copyright © 2006 Elsevier Ltd All rights reserved. GLC756 decreases TNF-α via an alpha2 and beta2 adrenoceptor related mechanismUlrich W. Laenglea, Anne U. Trendelenburgb, Rudolf Marksteina, Vicente Noguesa, Anne Provencher-Bollinger and Danielle Romana
- Dopamine D4-like receptors in untreated schizophrenic patients demonstrated with PET and 11C-SDZ GLC 756 European Neuropsychopharmacology, Volume 8, Supplement 2, November 1998, Page S235 A. Klimke, C. Boy, M. Eickhoff, H. Herzog, M. Holschbach, H. Mühlensiepen, M. Weckesser, E. Rota Kops, F. Sonnenberg, W. Gaebel, R. Markstein, G. Stöcklin, H. H. Coenen, H. W Müller-Gärtner
- Stefan, Martin (2002). An Atlas of Schizophrenia, p. 54
- Creese I, Burt DR, Snyder SH (1976). "Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs". Science (journal). 192 (4238): 481–3. PMID 3854.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Angrist, B; van Kammen, DP (1984). "CNS stimulants as a tool in the study of schizophrenia". Trends in Neurosciences. 7: 388–90. doi:10.1016/S0166-2236(84)80062-4.
- Lieberman JA, Kane JM, Alvir J (1987). "Provocative tests with psychostimulant drugs in schizophrenia". Psychopharmacology (Berl.). 91 (4): 415–33. doi:10.1007/BF00216006. PMID 2884687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Myers, David G. "Schizophrenia". Psychology. 2007. 678-85.
- Schizophrenia Research Volume 78, Issue 1, 1 October 2005, Pages 45-60 doi:10.1016/j.schres.2005.05.009 Copyright © 2005 Elsevier B.V. All rights reserved Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia C. Ervin Davisa, Dilip V. Jestea, and Lisa T. Eylera,
- http://www.acnp.org/g4/GN401000114/CH112.html
- E.M. Meisenzahl, G.J.Schmitt, J.Scheuerecker & H.-J. Moller, The role of dopamine for the pathophysiology of schizophrenia, Internationnal Review of Psychiatry, August 2007, 19(4)337-345
- Laruelle M (1998). "Imaging dopamine transmission in schizophrenia. A review and meta-analysis". Q J Nucl Med. 42 (3): 211–21. PMID 9796369.
{{cite journal}}: Unknown parameter|month=ignored (help) - Stone, James M.; Morrison, Paul D.; Pilowsky, Lyn S. (2006). "Review: Glutamate and dopamine dysregulation in schizophrenia a synthesis and selective review". Journal of Psychopharmacology. 21 (4): 440–52. doi:10.1177/0269881106073126. PMID 17259207.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Arch Gen Psychiatry. 1988 Jun;45(6):553-9. Dopamine metabolism and disposition in schizophrenic patients. Studies using debrisoquin. Maas JW, Contreras SA, Seleshi E, Bowden CL.
- Dopamine D2 densities and the schizophrenic brain Schizophrenia Research, Volume 32, Issue 3, 17 August 1998, Pages 201-206 Konstantine K. Zakzanis, Kevin T. Hansen
- Proc Natl Acad Sci U S A. 2000 July 5; 97(14): 7673–7675. PMCID: PMC33999 Copyright © 2000, The National Academy of Sciences Schizophrenia: More dopamine, more D2 receptors Philip Seeman* and Shitij Kapur†
- Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):8104-9.Increased baseline occupancy of D2 receptors by dopamine in schizophrenia.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M.
- Elevated D2/3-receptor availability in schizophrenia: A fallypride study Ingo Vernalekena, S.B. Eickhoffb, T. Veselinovica, M. Klompa, K. Spreckelmeyera, W. Schäferc and G. Gründera NeuroImage Volume 41, Supplement 2, 2008, Page T145
- Small Effect of Dopamine Release and No Effect of Dopamine Depletion on Fallypride Binding in Healthy Humans VANESSA L. CROPLEY,1,2 ROBERT B. INNIS,1 PRADEEP J. NATHAN,3 AMIRA K. BROWN,1 JANET L. SANGARE,1 ALICJA LERNER,1 YONG HOON RYU,1,4 KELLY E. SPRAGUE,1 VICTOR W. PIKE,1 AND MASAHIRO FUJITA SYNAPSE 62:399–408 (2008)
- Abi-Dargham, Anissa; Elsmarieke, E; Slifstein, Mark; Kegeles, Lawrence S.; Laruelle, Marc (2009). "Baseline and Amphetamine-Stimulated Dopamine Activity Are Related in Drug-Naïve Schizophrenic Subjects". Biological Psychiatry. 65 (12): 1091–1093. doi:10.1016/j.biopsych.2008.12.007. PMID 19167701.
- http://www.schizophreniaforum.org/new/detail.asp?id=1288 "Amphetamine psychosis has been proposed as a model for some features of schizophrenia... This model of amphetamine sensitization has also been adopted as a paradigm for researchers interested in the addictive powers of drugs of abuse."
- Bradley, Katherine C.; Meisel, Robert L. (2001). "Sexual Behavior Induction of c-Fos in the Nucleus Accumbens and Amphetamine-Stimulated Locomotor Activity Are Sensitized by Previous Sexual Experience in Female Syrian Hamsters". J. Neurosci. 21 (6): 2123–2130. PMID 11245696.
- Wong, Dean F. (1986). "Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics". Science. 234: 1558.
- Abi-Dargham A, Moore H (2003). "Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia". Neuroscientist. 9 (5): 404–16. doi:10.1177/1073858403252674. PMID 14580124.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Seeman P, Schwarz J, Chen JF; et al. (2006). "Psychosis pathways converge via D2high dopamine receptors". Synapse. 60 (4): 319–46. doi:10.1002/syn.20303. PMID 16786561.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Albert Hung Choy Wonga, Hubert H.M. Van Tol (2003) "Schizophrenia: from phenomenology to neurobiology" Neuroscience and Biobehavioral Reviews 27, p276 http://www.nature.com/nature/journal/v365/n6445/abs/365441a0.html
- for a discussion of opposing studies see p 143 PHILIP SEEMAN,*1 HONG-CHANG GUAN,1 JOSE NOBREGA,2 DILSHAD JIWA,2 RUDOLPH MARKSTEIN,3 JA-HYUN BALK,4 ROBERTO PICETTI,4 EMILIANA BORRELLI,4 AND HUBERT H.M. VAN TOL1, Dopamine D2-Like Sites in Schizophrenia, But Not in Alzheimer’s, Huntington’s, or Control Brains, for Benzquinoline SYNAPSE 25:137–146 (1997) http://www3.interscience.wiley.com/journal/52595/abstract?CRETRY=1&SRETRY=0
- Cooperativity in associating proteins. Monomer-dimer equilibrium coupled to ligand binding Alexander Levitzki, Joseph Schlessinger Biochemistry, 1974, 13 (25), pp 5214–5219 DOI: 10.1021/bi00722a026 Publication Date: December 1974 http://pubs.acs.org/doi/abs/10.1021/bi00722a026
- All Psychotic Roads Lead to Increased Dopamine D2High Receptors: A Perspective Journal Clinical Schizophrenia & Related Psychoses Publisher Walsh Medical Media ISSN 1935-1232 Issue Volume 1, Number 4 / January 2008 Category Translational Medicine DOI 10.3371/CSRP.1.4.7 Pages 351-355 http://clinicalschizophrenia.metapress.com/content/x32848665282821k/
- "Dopamine receptor pharmacology", Trends in Pharmacological Sciences, Volume 15, Issue 7, July 1994, Pages 264-270 Philip Seeman, Hubert H.M. Van Tol
- ^ Philip Seeman & H. B. Niznik (1990). "Dopamine receptors and transporters in Parkinson's disease and schizophrenia". FASEB Journal. 4: 2737–2744.
- Rote KV, Rechsteiner M (1986). "Degradation of proteins microinjected into HeLa cells. The role of substrate flexibility". J. Biol. Chem. 261 (33): 15430–6. PMID 2430958.
{{cite journal}}: Unknown parameter|month=ignored (help) - Konradi C, Heckers S (2003). "Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment". Pharmacol. Ther. 97 (2): 153–79. doi:10.1016/S0163-7258(02)00328-5. PMID 12559388.
{{cite journal}}: Unknown parameter|month=ignored (help) - Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA (2001). "Effects of ketamine in normal and schizophrenic volunteers". Neuropsychopharmacology. 25 (4): 455–67. doi:10.1016/S0893-133X(01)00243-3. PMID 11557159.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Coyle JT, Tsai G, Goff D (2003). "Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia". Ann. N. Y. Acad. Sci. 1003: 318–27. doi:10.1196/annals.1300.020. PMID 14684455.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Tuominen HJ, Tiihonen J, Wahlbeck K (2005). "Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis". Schizophr. Res. 72 (2–3): 225–34. doi:10.1016/j.schres.2004.05.005. PMID 15560967.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Yarlagadda A (2002). "Role of calcium regulation in pathophysiology model of schizophrenia and possible interventions". Med. Hypotheses. 58 (2): 182–6. doi:10.1054/mehy.2001.1511. PMID 11812200.
{{cite journal}}: Unknown parameter|month=ignored (help) - Yamada K, Ashikari I, Onishi K, Kanba S, Yagi G, Asai M (1995). "Effectiveness of nilvadipine in two cases of chronic schizophrenia". Psychiatry Clin. Neurosci. 49 (4): 237–8. doi:10.1111/j.1440-1819.1995.tb01891.x. PMID 9179944.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Prabakaran S, Swatton JE, Ryan MM; et al. (2004). "Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress". Mol. Psychiatry. 9 (7): 684–97, 643. doi:10.1038/sj.mp.4001511. PMID 15098003.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18408624, please use {{cite journal}} with
|pmid=18408624instead.