 | |
| Names | |
|---|---|
| Other names 1,1'-trithiaferrocenophane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider |
|
| PubChem CID |
|
SMILES
| |
| Properties | |
| Chemical formula | C10H8FeS3 |
| Molar mass | 280.20 g·mol |
| Appearance | Yellow solid |
| Density | 1.887 g/cm |
| Melting point | 149.5–150.5 °C (301.1–302.9 °F; 422.6–423.6 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
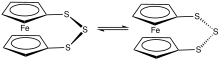
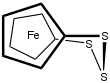
1,1'-Ferrocenetrisulfide is the organoiron compound with the formula Fe(C5H4S)2S. A yellow solid, it is the simplest polysulfide derivative of ferrocene. It can be synthesized by treatment of dilithioferrocene with elemental sulfur. Using proton NMR spectroscopy, the relatively slow conformational flexing of the trisulfide ring can be established.
References
- Davis, Betty R.; Bernal, Ivan (1972). "Structure of a novel fluxional molecule: 1,2,3-trithia--ferrocenophane". Journal of Crystal and Molecular Structure. 2 (3): 107–114. doi:10.1007/BF01464791. S2CID 96187454.
- Bishop, J.J.; Davison, A.; Katcher, M.L.; Lichtenberg, D.W.; Merrill, R.E.; Smart, J.C. (1971). "Symmetrically disubstituted ferrocenes". Journal of Organometallic Chemistry. 27 (2): 241–249. doi:10.1016/S0022-328X(00)80571-9.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |