 | |
| Names | |
|---|---|
| Other names 2-Amino-3-(5-fluoro-2,4-dioxopyrimidin-1-yl)propanoic acid | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.162.280 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H8FN3O4 |
| Molar mass | 217.156 g·mol |
| log P | -1.168 |
| Acidity (pKa) | 2.118 |
| Basicity (pKb) | 11.879 |
| Isoelectric point | 4.28 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used in vivo and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors in vitro. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato.
The name is unusual as it has two successive i's. This is not a typo.
Toxicity
(S)-5-Fluorowillardiine activity has been studied in vitro in a variety of neural tissues. In mouse embryo hippocampal neurons, it was found to desensitize AMPA/kainate receptors with an EC50 of 1.5 μM –— 7 times more potent than racemic AMPA (EC50 of 11 μM). In another study, (S)-5-Fluorowillardiine showed biphasic dose-dependent neurotoxicity in cultural rodent cortical neurons, with EC50 values of 0.70 and 170 μM. While in vivo research is sparse, a study in 5-day-old mice injected with the closely related AMPA/kainate agonist (S)-5-Bromowillardiine showed cortical and white matter damage. AMPA antagonists reduced the extent of the damage in a dose-dependent fashion.
Applications in research
Radiolabeled 5-fluorowillardiine has been used to study the distribution of ionotropic glutamate receptors in rodent brains. It has also been used to evaluate the effects of various allosteric modulators of the AMPA receptor.
Chemistry
Structure and activity
5-fluorowillardiine is derived from the nitrogenous base uracil found in RNA. It is one member of a family of willardiine compounds, which share uracil or a substituted uracil as an amino acid side chain. 5-Fluorowillardiine exists as two distinct isomers:
- (2R) or D
- (2S) or L
The particularly high affinity of 5-fluorowillardiine for the AMPA receptor is attributed to its fluorine substituent at the 5-position of the ring, which is electron-withdrawing and small enough to not interfere with binding. By contrast, related willardiine derivatives with larger nonpolar electron withdrawing groups exhibit greater affinity for kainate receptors than 5-fluorowillardiine, and less affinity for AMPA receptors.
The binding of 5-fluorowillardiine to the AMPA receptor is driven by entropy when its ring is uncharged. When the ring is deprotonated and has a negative charge, a favorable change in enthalpy primarily drives binding. Because the pKa values of halogenated willardiine derivates are approximately 8 (7.98 for 5-Fluorowillardiine), binding is mostly driven by an increase in entropy at physiological pH.
Synthesis
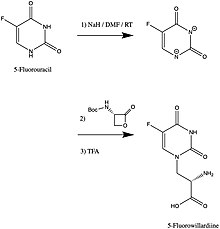
The synthesis of 5-Fluorowillardiine may be achieved by using 5-Fluorouracil as a nucleophile to open a specialized lactone in an SN2 reaction. Another straightforward approach is to perform a Strecker amino acid synthesis.
References
- Patneau, DK; Mayer, ML; Jane, DE; Watkins, JC (1992). "Activation and Desensitization of AMPA / Kainate Receptors by Novel Derivatives of Willardiine". Journal of Neuroscience. 12 (2): 595–606. doi:10.1523/JNEUROSCI.12-02-00595.1992. PMC 6575614. PMID 1371315.
- Hawkins, LM; Beaver, KM; Jane, DE; Taylor, PM; Sunter, DC; Roberts, PJ (1995). "Characterization of the pharmacology and regional distribution of (S)-H-5-fluorowillardiine binding in rat brain". British Journal of Pharmacology. 116 (3): 2033–9. doi:10.1111/j.1476-5381.1995.tb16408.x. PMC 1908955. PMID 8640342.
- Lunn, ML; Ganakas, AM; Mercer, LD; Lawrence, AJ; Beart, PM (1996). "Localisation and properties of AMPA-insensitive kainate sites: receptor autoradiography and gene expression in rat brain". Neuroscience Letters. 204 (1–2): 121–4. doi:10.1016/0304-3940(96)12335-1. PMID 8929993. S2CID 36885666.
- Larm, JA; Cheung, NS; Beart, PM (1996). "(S)-5-fluorowillardiine-mediated neurotoxicity in cultured murine cortical neurones occurs via AMPA and kainate receptors". European Journal of Pharmacology. 314 (1–2): 249–54. doi:10.1016/S0014-2999(96)00633-4. PMID 8957243.
- Jensen, RJ (1999). "Responses of directionally selective retinal ganglion cells to activation of AMPA glutamate receptors". Visual Neuroscience. 16 (2): 205–19. doi:10.1017/s0952523899162023. PMID 10367956. S2CID 42955027.
- Olivera, S; Rodriguez-Ithurralde, D; Henley, JM (2001). "Regional localization and developmental profile of acetylcholinesterase-evoked increases in H-5-fluorowillardiine binding to AMPA receptors in rat brain". British Journal of Pharmacology. 133 (7): 1055–62. doi:10.1038/sj.bjp.0704167. PMC 1572873. PMID 11487516.
- Kessler, M; Arai, AC (2006). "Use of H fluorowillardiine to study properties of AMPA receptor allosteric modulators". Brain Research. 1076 (1): 25–41. doi:10.1016/j.brainres.2005.09.024. PMID 16256076. S2CID 28267484.
- Klaassen, C. D.; John Barr Watkins (2010). "Toxic Agents" (PDF). Casarett and Doull's essentials of toxicology. USA: McGraw-Hill Prof Med/Tech. p. 374. ISBN 978-0-07-176651-7.
- Atta-ur- Rahman (2000). "Interference of Alkaloids" (PDF). Bioactive Natural Products (Part B), Part 2. Amsterdam: Alsevier Science B. V. p. 72. ISBN 9780080542010.
- Patneau, DK; Mayer, ML; Jane, DE; Watkins, JC (1 February 1992). "Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine". The Journal of Neuroscience. 12 (2): 595–606. doi:10.1523/JNEUROSCI.12-02-00595.1992. PMC 6575614. PMID 1371315.
- Larm, Jari A.; Cheung, Nam Sang; Beart, Philip M. (October 1996). "(S)-5-Fluorowillardiine-mediated neurotoxicity in cultured murine cortical neurones occurs via AMPA and kainate receptors". European Journal of Pharmacology. 314 (1–2): 249–254. doi:10.1016/S0014-2999(96)00633-4. PMID 8957243.
- Gressens, Pierre; Spedding, Michael; Gigler, Gabor; Kertesz, Szabolcs; Villa, Pascal; Medja, Fadia; Williamson, Toni; Kapus, Gabor; Levay, Gyorgy; Szenasi, Gabor; Barkoczy, Jozsef; Harsing, Laszlo G. (September 2005). "The effects of AMPA receptor antagonists in models of stroke and neurodegeneration". European Journal of Pharmacology. 519 (1–2): 58–67. doi:10.1016/j.ejphar.2005.06.031. PMID 16112106.
- Hawkins, L.M.; Beaver, K.M.; Jane, D.E.; Taylor, P.M.; Sunter, D.C.; Roberts, P.J. (October 1995). "Characterization of the pharmacology and regional distribution of (S)-[3H]-5-fluorowillardiine binding in rat brain". British Journal of Pharmacology. 116 (3): 2033–2039. doi:10.1111/j.1476-5381.1995.tb16408.x. PMC 1908955. PMID 8640342.
- Kessler, Markus; Arai, Amy C. (March 2006). "Use of fluorowillardiine to study properties of AMPA receptor allosteric modulators". Brain Research. 1076 (1): 25–41. doi:10.1016/j.brainres.2005.09.024. PMID 16256076. S2CID 28267484.
- Jane, David E.; Hoo, Ken; Kamboj, Raj; Deverill, Michele; Bleakman, David; Mandelzys, Allan (October 1997). "Synthesis of Willardiine and 6-Azawillardiine Analogs: Pharmacological Characterization on Cloned Homomeric Human AMPA and Kainate Receptor Subtypes". Journal of Medicinal Chemistry. 40 (22): 3645–3650. doi:10.1021/jm9702387. PMID 9357531.
- Martinez, Madeline; Ahmed, Ahmed H.; Loh, Adrienne P.; Oswald, Robert E. (5 June 2014). "Thermodynamics and Mechanism of the Interaction of Willardiine Partial Agonists with a Glutamate Receptor: Implications for Drug Development". Biochemistry. 53 (23): 3790–3795. doi:10.1021/bi500511m. PMC 4215890. PMID 24850223.
- Jane, David E.; Hoo, Ken; Kamboj, Raj; Deverill, Michele; Bleakman, David; Mandelzys, Allan (October 1997). "Synthesis of Willardiine and 6-Azawillardiine Analogs: Pharmacological Characterization on Cloned Homomeric Human AMPA and Kainate Receptor Subtypes". Journal of Medicinal Chemistry. 40 (22): 3645–3650. doi:10.1021/jm9702387. PMID 9357531.
- Dewar, J. H.; Shaw, G. (1962). "110. Purines, pyrimidines, and imidazoles. Part XVII. A synthesis of willardiine". Journal of the Chemical Society (Resumed): 583. doi:10.1039/JR9620000583.