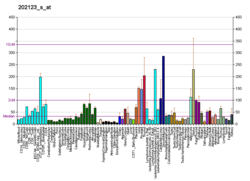
Tyrosine-protein kinase ABL1 also known as ABL1 is a protein that, in humans, is encoded by the ABL1 gene (previous symbol ABL) located on chromosome 9. c-Abl is sometimes used to refer to the version of the gene found within the mammalian genome, while v-Abl refers to the viral gene, which was initially isolated from the Abelson murine leukemia virus.
Function
The ABL1 proto-oncogene encodes a cytoplasmic and nuclear protein tyrosine kinase that has been implicated in processes of cell differentiation, cell division, cell adhesion, and stress response such as DNA repair. Activity of ABL1 protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns ABL1 into an oncogene. The t(9;22) translocation results in the head-to-tail fusion of the BCR and ABL1 genes, leading to a fusion gene present in many cases of chronic myelogenous leukemia. The DNA-binding activity of the ubiquitously expressed ABL1 tyrosine kinase is regulated by CDC2-mediated phosphorylation, suggesting a cell cycle function for ABL1. The ABL1 gene is expressed as either a 6- or a 7-kb mRNA transcript, with alternatively spliced first exons spliced to the common exons 2–11.
Clinical significance

Mutations in the ABL1 gene are associated with chronic myelogenous leukemia (CML). In CML, the gene is activated by being translocated within the BCR (breakpoint cluster region) gene on chromosome 22. This new fusion gene, BCR-ABL, encodes an unregulated, cytoplasm-targeted tyrosine kinase that allows the cells to proliferate without being regulated by cytokines. This, in turn, allows the cell to become cancerous.
This gene is a partner in a fusion gene with the BCR gene in the Philadelphia chromosome, a characteristic abnormality in chronic myelogenous leukemia (CML) and rarely in some other leukemia forms. The BCR-ABL transcript encodes a tyrosine kinase, which activates mediators of the cell cycle regulation system, leading to a clonal myeloproliferative disorder. The BCR-ABL protein can be inhibited by various small molecules. One such inhibitor is imatinib mesylate, which occupies the tyrosine kinase domain and inhibits BCR-ABL's influence on the cell cycle. Second generation BCR-ABL tyrosine-kinase inhibitors are also under development to inhibit BCR-ABL mutants resistant to imatinib.
Interactions
ABL gene has been shown to interact with:
- ABI1,
- ABI2,
- ABL2,
- ATM,
- BCAR1,
- BCR,
- BRCA1,
- CAT,
- CBL,
- CRKL,
- DOK1,
- EPHB2,
- GPX1,
- GRB10,
- MTOR,
- GRB2,
- MDM2,
- NCK1,
- NEDD9,
- NTRK1,
- P73,
- PAG1,
- PAK2,
- PSTPIP1,
- RAD9A,
- RAD51,
- RB1,
- RFX1,
- RYBP,
- SHC1,
- SORBS2,
- SPTA1,
- SPTAN1,
- TERF1,
- VAV1, and
- YTHDC1.
Regulation
There is some evidence that the expression of Abl is regulated by the microRNA miR-203.
See also
References
- ^ GRCh38: Ensembl release 89: ENSG00000097007 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000026842 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Szczylik C, Skorski T, Nicolaides NC, Manzella L, Malaguarnera L, Venturelli D, Gewirtz AM, Calabretta B (August 1991). "Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides". Science. 253 (5019): 562–5. Bibcode:1991Sci...253..562S. doi:10.1126/science.1857987. PMID 1857987.
- Abelson HT, Rabstein LS (August 1970). "Lymphosarcoma: virus-induced thymic-independent disease in mice". Cancer Research. 30 (8): 2213–22. PMID 4318922.
- Takizawa Y, Kinebuchi T, Kagawa W, Yokoyama S, Shibata T, Kurumizaka H (September 2004). "Mutational analyses of the human Rad51-Tyr315 residue, a site for phosphorylation in leukaemia cells". Genes to Cells. 9 (9): 781–90. doi:10.1111/j.1365-2443.2004.00772.x. PMID 15330855. S2CID 22916981.
- Salles D, Mencalha AL, Ireno IC, Wiesmüller L, Abdelhay E (January 2011). "BCR-ABL stimulates mutagenic homologous DNA double-strand break repair via the DNA-end-processing factor CtIP". Carcinogenesis. 32 (1): 27–34. doi:10.1093/carcin/bgq216. PMID 20974687.
- Siddiqui A, Tumiati M, Joko A, Sandholm J, Roering P, Aakko S, et al. (2021). "Targeting DNA Homologous Repair Proficiency With Concomitant Topoisomerase II and c-Abl Inhibition". Frontiers in Oncology. 11: 3666. doi:10.3389/fonc.2021.733700. PMC 8488401. PMID 34616682.
- "UniProtKB - P00519 (ABL1_HUMAN)". Uniprot. Retrieved 18 May 2020.
- "Entrez Gene: ABL1 v-abl Abelson murine leukemia viral oncogene homolog 1".
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL (July 2004). "Overriding imatinib resistance with a novel ABL kinase inhibitor". Science. 305 (5682): 399–401. Bibcode:2004Sci...305..399S. doi:10.1126/science.1099480. PMID 15256671. S2CID 34972913.
- Tani K, Sato S, Sukezane T, Kojima H, Hirose H, Hanafusa H, Shishido T (June 2003). "Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase". J. Biol. Chem. 278 (24): 21685–92. doi:10.1074/jbc.M301447200. PMID 12672821.
- Biesova Z, Piccoli C, Wong WT (January 1997). "Isolation and characterization of e3B1, an eps8 binding protein that regulates cell growth". Oncogene. 14 (2): 233–41. doi:10.1038/sj.onc.1200822. PMID 9010225. S2CID 22964580.
- Yamamoto A, Suzuki T, Sakaki Y (June 2001). "Isolation of hNap1BP which interacts with human Nap1 (NCKAP1) whose expression is down-regulated in Alzheimer's disease". Gene. 271 (2): 159–69. doi:10.1016/S0378-1119(01)00521-2. PMID 11418237.
- ^ Cao C, Leng Y, Li C, Kufe D (April 2003). "Functional interaction between the c-Abl and Arg protein-tyrosine kinases in the oxidative stress response". J. Biol. Chem. 278 (15): 12961–7. doi:10.1074/jbc.M300058200. PMID 12569093.
- Dai Z, Pendergast AM (November 1995). "Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity". Genes Dev. 9 (21): 2569–82. doi:10.1101/gad.9.21.2569. PMID 7590236.
- ^ Chen G, Yuan SS, Liu W, Xu Y, Trujillo K, Song B, Cong F, Goff SP, Wu Y, Arlinghaus R, Baltimore D, Gasser PJ, Park MS, Sung P, Lee EY (April 1999). "Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl". J. Biol. Chem. 274 (18): 12748–52. doi:10.1074/jbc.274.18.12748. PMID 10212258.
- Shafman T, Khanna KK, Kedar P, Spring K, Kozlov S, Yen T, Hobson K, Gatei M, Zhang N, Watters D, Egerton M, Shiloh Y, Kharbanda S, Kufe D, Lavin MF (May 1997). "Interaction between ATM protein and c-Abl in response to DNA damage". Nature. 387 (6632): 520–3. Bibcode:1997Natur.387R.520S. doi:10.1038/387520a0. PMID 9168117. S2CID 4334242.
- ^ Kishi S, Zhou XZ, Ziv Y, Khoo C, Hill DE, Shiloh Y, Lu KP (August 2001). "Telomeric protein Pin2/TRF1 as an important ATM target in response to double strand DNA breaks". J. Biol. Chem. 276 (31): 29282–91. doi:10.1074/jbc.M011534200. PMID 11375976.
- Salgia R, Pisick E, Sattler M, Li JL, Uemura N, Wong WK, Burky SA, Hirai H, Chen LB, Griffin JD (October 1996). "p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene". J. Biol. Chem. 271 (41): 25198–203. doi:10.1074/jbc.271.41.25198. PMID 8810278.
- Mayer BJ, Hirai H, Sakai R (March 1995). "Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases". Curr. Biol. 5 (3): 296–305. Bibcode:1995CBio....5..296M. doi:10.1016/S0960-9822(95)00060-1. PMID 7780740. S2CID 16957239.
- ^ Puil L, Liu J, Gish G, Mbamalu G, Bowtell D, Pelicci PG, Arlinghaus R, Pawson T (February 1994). "Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway". EMBO J. 13 (4): 764–73. doi:10.1002/j.1460-2075.1994.tb06319.x. PMC 394874. PMID 8112292.
- Ling X, Ma G, Sun T, Liu J, Arlinghaus RB (January 2003). "Bcr and Abl interaction: oncogenic activation of c-Abl by sequestering Bcr". Cancer Res. 63 (2): 298–303. PMID 12543778.
- Pendergast AM, Muller AJ, Havlik MH, Maru Y, Witte ON (July 1991). "BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner". Cell. 66 (1): 161–71. doi:10.1016/0092-8674(91)90148-R. PMID 1712671. S2CID 9933891.
- Foray N, Marot D, Randrianarison V, Venezia ND, Picard D, Perricaudet M, Favaudon V, Jeggo P (June 2002). "Constitutive association of BRCA1 and c-Abl and its ATM-dependent disruption after irradiation". Mol. Cell. Biol. 22 (12): 4020–32. doi:10.1128/MCB.22.12.4020-4032.2002. PMC 133860. PMID 12024016.
- Cao C, Leng Y, Kufe D (August 2003). "Catalase activity is regulated by c-Abl and Arg in the oxidative stress response". J. Biol. Chem. 278 (32): 29667–75. doi:10.1074/jbc.M301292200. PMID 12777400.
- ^ Miyoshi-Akiyama T, Aleman LM, Smith JM, Adler CE, Mayer BJ (July 2001). "Regulation of Cbl phosphorylation by the Abl tyrosine kinase and the Nck SH2/SH3 adaptor". Oncogene. 20 (30): 4058–69. doi:10.1038/sj.onc.1204528. PMID 11494134.
- ^ Soubeyran P, Barac A, Szymkiewicz I, Dikic I (February 2003). "Cbl-ArgBP2 complex mediates ubiquitination and degradation of c-Abl". Biochem. J. 370 (Pt 1): 29–34. doi:10.1042/BJ20021539. PMC 1223168. PMID 12475393.
- ^ Ren R, Ye ZS, Baltimore D (April 1994). "Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites". Genes Dev. 8 (7): 783–95. doi:10.1101/gad.8.7.783. PMID 7926767.
- Heaney C, Kolibaba K, Bhat A, Oda T, Ohno S, Fanning S, Druker BJ (January 1997). "Direct binding of CRKL to BCR-ABL is not required for BCR-ABL transformation". Blood. 89 (1): 297–306. doi:10.1182/blood.V89.1.297. PMID 8978305.
- Kyono WT, de Jong R, Park RK, Liu Y, Heisterkamp N, Groffen J, Durden DL (November 1998). "Differential interaction of Crkl with Cbl or C3G, Hef-1, and gamma subunit immunoreceptor tyrosine-based activation motif in signaling of myeloid high affinity Fc receptor for IgG (Fc gamma RI)". J. Immunol. 161 (10): 5555–63. doi:10.4049/jimmunol.161.10.5555. PMID 9820532.
- van Dijk TB, van Den Akker E, Amelsvoort MP, Mano H, Löwenberg B, von Lindern M (November 2000). "Stem cell factor induces phosphatidylinositol 3'-kinase-dependent Lyn/Tec/Dok-1 complex formation in hematopoietic cells". Blood. 96 (10): 3406–13. doi:10.1182/blood.V96.10.3406. hdl:1765/9530. PMID 11071635.
- Yamanashi Y, Baltimore D (January 1997). "Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok". Cell. 88 (2): 205–11. doi:10.1016/S0092-8674(00)81841-3. PMID 9008161. S2CID 14205526.
- Yu HH, Zisch AH, Dodelet VC, Pasquale EB (July 2001). "Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor". Oncogene. 20 (30): 3995–4006. doi:10.1038/sj.onc.1204524. PMID 11494128. S2CID 25752193.
- Cao C, Leng Y, Huang W, Liu X, Kufe D (October 2003). "Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases". J. Biol. Chem. 278 (41): 39609–14. doi:10.1074/jbc.M305770200. PMID 12893824.
- Bai RY, Jahn T, Schrem S, Munzert G, Weidner KM, Wang JY, Duyster J (August 1998). "The SH2-containing adapter protein GRB10 interacts with BCR-ABL". Oncogene. 17 (8): 941–8. doi:10.1038/sj.onc.1202024. PMID 9747873. S2CID 20866214.
- Frantz JD, Giorgetti-Peraldi S, Ottinger EA, Shoelson SE (January 1997). "Human GRB-IRbeta/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains". J. Biol. Chem. 272 (5): 2659–67. doi:10.1074/jbc.272.5.2659. PMID 9006901.
- Kumar V, Sabatini D, Pandey P, Gingras AC, Majumder PK, Kumar M, Yuan ZM, Carmichael G, Weichselbaum R, Sonenberg N, Kufe D, Kharbanda S (April 2000). "Regulation of the rapamycin and FKBP-target 1/mammalian target of rapamycin and cap-dependent initiation of translation by the c-Abl protein-tyrosine kinase". J. Biol. Chem. 275 (15): 10779–87. doi:10.1074/jbc.275.15.10779. PMID 10753870.
- Warmuth M, Bergmann M, Priess A, Häuslmann K, Emmerich B, Hallek M (December 1997). "The Src family kinase Hck interacts with Bcr-Abl by a kinase-independent mechanism and phosphorylates the Grb2-binding site of Bcr". J. Biol. Chem. 272 (52): 33260–70. doi:10.1074/jbc.272.52.33260. PMID 9407116.
- Goldberg Z, Vogt Sionov R, Berger M, Zwang Y, Perets R, Van Etten RA, Oren M, Taya Y, Haupt Y (July 2002). "Tyrosine phosphorylation of Mdm2 by c-Abl: implications for p53 regulation". EMBO J. 21 (14): 3715–27. doi:10.1093/emboj/cdf384. PMC 125401. PMID 12110584.
- Minegishi M, Tachibana K, Sato T, Iwata S, Nojima Y, Morimoto C (October 1996). "Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta 1 integrin-mediated signaling in lymphocytes". J. Exp. Med. 184 (4): 1365–75. doi:10.1084/jem.184.4.1365. PMC 2192828. PMID 8879209.
- Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA (July 1996). "Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae". Mol. Cell. Biol. 16 (7): 3327–37. doi:10.1128/mcb.16.7.3327. PMC 231327. PMID 8668148.
- Koch A, Mancini A, Stefan M, Niedenthal R, Niemann H, Tamura T (March 2000). "Direct interaction of nerve growth factor receptor, TrkA, with non-receptor tyrosine kinase, c-Abl, through the activation loop". FEBS Lett. 469 (1): 72–6. doi:10.1016/S0014-5793(00)01242-4. PMID 10708759.
- Yano H, Cong F, Birge RB, Goff SP, Chao MV (February 2000). "Association of the Abl tyrosine kinase with the Trk nerve growth factor receptor". J. Neurosci. Res. 59 (3): 356–64. doi:10.1002/(SICI)1097-4547(20000201)59:3<356::AID-JNR9>3.0.CO;2-G. PMID 10679771. S2CID 10977765.
- Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, Lu H, Kharbanda S, Weichselbaum R, Kufe D (June 1999). "p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage". Nature. 399 (6738): 814–7. Bibcode:1999Natur.399..814Y. doi:10.1038/21704. PMID 10391251. S2CID 4421613.
- Agami R, Blandino G, Oren M, Shaul Y (June 1999). "Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis". Nature. 399 (6738): 809–13. Bibcode:1999Natur.399..809A. doi:10.1038/21697. PMID 10391250. S2CID 4394015.
- Wen ST, Van Etten RA (October 1997). "The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity". Genes Dev. 11 (19): 2456–67. doi:10.1101/gad.11.19.2456. PMC 316562. PMID 9334312.
- Roig J, Tuazon PT, Zipfel PA, Pendergast AM, Traugh JA (December 2000). "Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK". Proc. Natl. Acad. Sci. U.S.A. 97 (26): 14346–51. Bibcode:2000PNAS...9714346R. doi:10.1073/pnas.97.26.14346. PMC 18921. PMID 11121037.
- Cong F, Spencer S, Côté JF, Wu Y, Tremblay ML, Lasky LA, Goff SP (December 2000). "Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation". Mol. Cell. 6 (6): 1413–23. doi:10.1016/S1097-2765(00)00138-6. PMID 11163214.
- Yoshida K, Komatsu K, Wang HG, Kufe D (May 2002). "c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage". Mol. Cell. Biol. 22 (10): 3292–300. doi:10.1128/MCB.22.10.3292-3300.2002. PMC 133797. PMID 11971963.
- Miyamura T, Nishimura J, Yufu Y, Nawata H (February 1997). "Interaction of BCR-ABL with the retinoblastoma protein in Philadelphia chromosome-positive cell lines". Int. J. Hematol. 65 (2): 115–21. doi:10.1016/S0925-5710(96)00539-7. PMID 9071815.
- Welch PJ, Wang JY (November 1993). "A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle". Cell. 75 (4): 779–90. doi:10.1016/0092-8674(93)90497-E. PMID 8242749.
- Agami R, Shaul Y (April 1998). "The kinase activity of c-Abl but not v-Abl is potentiated by direct interaction with RFXI, a protein that binds the enhancers of several viruses and cell-cycle regulated genes". Oncogene. 16 (14): 1779–88. doi:10.1038/sj.onc.1201708. PMID 9583676.
- Zhu J, Shore SK (December 1996). "c-ABL tyrosine kinase activity is regulated by association with a novel SH3-domain-binding protein". Mol. Cell. Biol. 16 (12): 7054–62. doi:10.1128/mcb.16.12.7054. PMC 231708. PMID 8943360.
- Wisniewski D, Strife A, Swendeman S, Erdjument-Bromage H, Geromanos S, Kavanaugh WM, Tempst P, Clarkson B (April 1999). "A novel SH2-containing phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase (SHIP2) is constitutively tyrosine phosphorylated and associated with src homologous and collagen gene (SHC) in chronic myelogenous leukemia progenitor cells". Blood. 93 (8): 2707–20. doi:10.1182/blood.V93.8.2707. PMID 10194451.
- Wang B, Golemis EA, Kruh GD (July 1997). "ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl-interacting protein, is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks". J. Biol. Chem. 272 (28): 17542–50. doi:10.1074/jbc.272.28.17542. hdl:20.500.12613/9174. PMID 9211900.
- ^ Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L (May 1998). "Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton". J. Biol. Chem. 273 (22): 13681–92. doi:10.1074/jbc.273.22.13681. PMID 9593709.
- Bassermann F, Jahn T, Miething C, Seipel P, Bai RY, Coutinho S, Tybulewicz VL, Peschel C, Duyster J (April 2002). "Association of Bcr-Abl with the proto-oncogene Vav is implicated in activation of the Rac-1 pathway". J. Biol. Chem. 277 (14): 12437–45. doi:10.1074/jbc.M112397200. PMID 11790798.
- Rafalska I, Zhang Z, Benderska N, Wolff H, Hartmann AM, Brack-Werner R, Stamm S (August 2004). "The intranuclear localization and function of YT521-B is regulated by tyrosine phosphorylation". Hum. Mol. Genet. 13 (15): 1535–49. doi:10.1093/hmg/ddh167. PMID 15175272.
- Bueno MJ, Pérez de Castro I, Gómez de Cedrón M, Santos J, Calin GA, Cigudosa JC, Croce CM, Fernández-Piqueras J, Malumbres M (June 2008). "Genetic and epigenetic silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene expression". Cancer Cell. 13 (6): 496–506. doi:10.1016/j.ccr.2008.04.018. hdl:10261/7369. PMID 18538733. (Erratum: doi:10.1016/j.ccell.2016.03.013, PMID 27070707)
Further reading
- Shore SK, Tantravahi RV, Reddy EP (December 2002). "Transforming pathways activated by the v-Abl tyrosine kinase". Oncogene. 21 (56): 8568–76. doi:10.1038/sj.onc.1206084. PMID 12476303. S2CID 42502628.
- Shaul Y (2000). "c-Abl: activation and nuclear targets". Cell Death Differ. 7 (1): 10–6. doi:10.1038/sj.cdd.4400626. PMID 10713716.
- Era T (2002). "Bcr-Abl is a "molecular switch" for the decision for growth and differentiation in hematopoietic stem cells". Int. J. Hematol. 76 (1): 35–43. doi:10.1007/BF02982716. PMID 12138893. S2CID 10269867.
- Pendergast AM (2002). "The Abl family kinases: mechanisms of regulation and signaling". Advances in Cancer Research Volume 85. Vol. 85. pp. 51–100. doi:10.1016/S0065-230X(02)85003-5. ISBN 978-0-12-006685-8. PMID 12374288.
- Keung YK, Beaty M, Steward W, Jackle B, Pettnati M (2002). "Chronic myelocytic leukemia with eosinophilia, t(9;12)(q34;p13), and ETV6-ABL gene rearrangement: case report and review of the literature". Cancer Genet. Cytogenet. 138 (2): 139–42. doi:10.1016/S0165-4608(02)00609-X. PMID 12505259.
- Saglio G, Cilloni D (2004). "Abl: the prototype of oncogenic fusion proteins". Cell. Mol. Life Sci. 61 (23): 2897–911. doi:10.1007/s00018-004-4271-0. PMID 15583852. S2CID 35998018.
- Shaul Y, Ben-Yehoyada M (2005). "Role of c-Abl in the DNA damage stress response". Cell Res. 15 (1): 33–5. doi:10.1038/sj.cr.7290261. PMID 15686624.
- Yoshida K (2007). "Regulation for Nuclear Targeting of the Abl Tyrosine Kinase in Response to DNA Damage". Advances in Molecular Oncology. Advances in Experimental Medicine and Biology. Vol. 604. Springer. pp. 155–65. doi:10.1007/978-0-387-69116-9_15. ISBN 978-0-387-69114-5. PMID 17695727.
External links
- Genes,+abl at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Online Mendelian Inheritance in Man (OMIM): 189980 (ABL)
- Abelson+Leukemia+Virus at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Drosophila Abl tyrosine kinase - The Interactive Fly
- ABL1 Info with links in the Cell Migration Gateway
- ABL1 on the Atlas of Genetics and Oncology
- Human ABL1 genome location and ABL1 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P00519 (Human Tyrosine-protein kinase ABL1) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: P00520 (Mouse Tyrosine-protein kinase ABL1) at the PDBe-KB.
| PDB gallery | |
|---|---|
|
| Protein kinases: tyrosine kinases (EC 2.7.10) | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|