 | |
 | |
| Names | |
|---|---|
| IUPAC name hexanedial | |
| Other names 1,4-Butane dicarboxaldehyde, 1,6-hexanedial | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.731 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
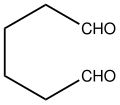
| Chemical formula | C6H10O2 |
| Molar mass | 114.144 g·mol |
| Appearance | colorless liquid |
| Density | 1.003 g/cm |
| Melting point | −8 °C (18 °F; 265 K) |
| Boiling point | 68–70 °C (154–158 °F; 341–343 K) 3 mm Hg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Adipaldehyde is the organic compound with the formula (CH2)4(CHO)2. It is a colorless oil that is usually encountered as an aqueous solution because it is highly reactive like many dialdehydes. The compound has attracted interest as a precursor to nylon-related polymers. It can be produced by double hydroformylation of 1,3-butadiene, but this methodology has not achieved commercialization. It has been prepared by oxidation of 1,6-hexanediol with pyridinium chlorochromate.
References
- Hardy, P. M.; Nicholls, A. C.; Rydon, H. N. (1972). "The Hydration and Polymerisation of Succinaldehyde, Glutaraldehyde, and Adipaldehyde". Journal of the Chemical Society, Perkin Transactions 2 (15): 2270. doi:10.1039/P29720002270.
- Yu, Si-min; Snavely, William K.; Chaudhari, Raghunath V.; Subramaniam, Bala (2020). "Butadiene hydroformylation to adipaldehyde with Rh-based catalysts: Insights into ligand effects". Molecular Catalysis. 484. doi:10.1016/j.mcat.2019.110721. S2CID 213312335.
- Corey, E.J.; Suggs, J.William (1975). "Pyridinium Chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds". Tetrahedron Letters. 16 (31): 2647–2650. doi:10.1016/s0040-4039(00)75204-x.