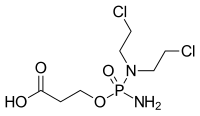
 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-({Aminophosphoryl}oxy)propanoic acid | |
| Other names Carboxyphosphamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H15Cl2N2O4P |
| Molar mass | 293.084762 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Carboxycyclophosphamide is an inactive metabolite of the cytotoxic antineoplastic drug cyclophosphamide. In the metabolic pathway of cyclophosphamide inactivation it first metabolizes to 4-hydroxycyclophosphamide, then partially tautomerizes into aldophosphamide. Aldophosphamide then, in turn, is oxidized into carboxycyclophosphamide by the enzyme ALDH (aldehyde dehydrogenase).
References
- Dockham, PA; Lee, MO; Sladek, NE (1992). "Identification of human liver aldehyde dehydrogenases that catalyze the oxidation of aldophosphamide and retinaldehyde". Biochem Pharmacol. 43 (11): 2453–69. doi:10.1016/0006-2952(92)90326-e. PMID 1610409.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |