 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6ClNO2 |
| Molar mass | 123.54 g·mol |
| Appearance | White solid |
| Melting point | 166–167 °C (331–333 °F; 439–440 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
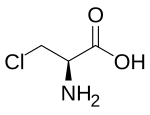
Chloroalanine (3-chloroalanine) is an unnatural amino acid with the formula ClCH2CH(NH2)CO2H. It is a white, water-soluble solid. The compound is usually derived from chlorination of serine. The compound is used in the synthesis of other amino acids by replacement of the chloride. Protected forms of the related iodoalanine are also known.
Chemical properties
The hydrolysis of 3-chloro-D-alanine is catalyzed by the enzyme 3-chloro-D-alanine dehydrochlorinase:
- ClCH2CH(NH2)CO2H + H2O →CH3C(O)CO2H + NH4Cl
References
- Hondal, Robert J.; Nilsson, Bradley L.; Raines, Ronald T. (2001). "Selenocysteine in Native Chemical Ligation and Expressed Protein Ligation". Journal of the American Chemical Society. 123 (21): 5140–5141. doi:10.1021/ja005885t. PMID 11457362.
- Richard F. W. Jackson; Manuel Perez-Gonzalez (2005). "Synthesis of N-(Tert-butoxycarbonyl)-β-iodoalanine Methyl Ester: A Useful Building Block in the Synthesis of Nonnatural α-amino Acids via Palladium Catalyzed Cross Coupling Reactions". Org. Synth. 81: 77. doi:10.15227/orgsyn.081.0077.
- Atmuri, N. D. P.; Lubell, W. D. (2015). "Preparation of N-(Boc)-Allylglycine Methyl Ester Using a Zinc-mediated, Palladium-catalyzed Cross-coupling Reaction". Org. Synth. 92: 103. doi:10.15227/orgsyn.092.0103.
- Yamada H, Nagasawa T, Ohkishi H, Kawakami B, Tani Y (June 1981). "Synthesis of D-cysteine from 3-chloro-D-alanine and hydrogen sulfide by 3-chloro-D-alanine hydrogen chloride-lyase (deaminating) of Pseudomonas putida". Biochemical and Biophysical Research Communications. 100 (3): 1104–10. doi:10.1016/0006-291X(81)91937-9. PMID 6791643.