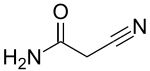
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Cyanoacetamide | |
| Other names
Malonamide nitrile 3-Nitrilopropionamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.003.211 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H4N2O |
| Molar mass | 84.078 g·mol |
| Density | 1.163 g/cm |
| Melting point | 119 to 121 °C (246 to 250 °F; 392 to 394 K) |
| Boiling point | 351.2 °C (664.2 °F; 624.3 K) |
| Acidity (pKa) | ca. 11 13.24 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2-Cyanoacetamide is an organic compound. It is an acetic amide with a nitrile functional group.
Uses
Cyanoacetamide is used in spectrofluorimetric methods to determine the activity of antihistamine H1 receptor antagonistic drugs such as ebastine, cetirizine dihydrochloride and fexofenadine hydrochloride.
Preparation
2-Cyanoacetamide is prepared from chloroacetic acid via Kolbe nitrile synthesis followed by Fischer esterification and ester aminolysis.
See also
References
- George H. Schenk (Jun 23, 2016). Organic Functional Group Analysis: Theory and Development. Elsevier. ISBN 9781483136073.
- Jay Sung; Si-Ying Hsu; Tzu-Hua Wang; Amanda Pan; Andrew Yeh (2006). "Kinetic studies of the reactions of pentacyanonitrosylferrate(2−) with ligands containing acidic methylene groups". Inorganica Chimica Acta. 359 (12): 3888–3894. doi:10.1016/j.ica.2006.04.042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Ibrahim, F.; Sharaf El-Din, M. K.; Eid, M.; Wahba, M. E. K. (2011). "Spectrofluorimetric Determination Of Some H1 Receptor Antagonist Drugs In Pharmaceutical Formulations And Biological Fluids". International Journal of Pharmaceutical Sciences and Research. 21 (8): 2056–2072. doi:10.13040/IJPSR.0975-8232.2(8).2056-72.
- Inglis, J. K. H. (1928). "Ethyl Cyanoacetate". Organic Syntheses. 8: 74. doi:10.15227/orgsyn.008.0074.
- Corson, B. B.; Scott, R. W.; Vose, C. E. (1941). "Cyanoacetamide". Organic Syntheses. 1: 179. doi:10.15227/orgsyn.009.0036.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |