 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.148 |
| Chemical and physical data | |
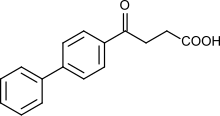
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol |
| 3D model (JSmol) | |
| Melting point | 186 °C (367 °F) |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Fenbufen is a nonsteroidal anti-inflammatory drug used to treat pain.
Fenbufen is a member of the propionic acid derivatives class of drugs.
It was introduced by American Cyanamid under the trade name Lederfen in the 1980s. Due to liver toxicity, it was withdrawn from markets in the developed world in 2010.
As of 2015 it was available in Taiwan and Thailand under several brand names.
Preparation
Fenbufen can be synthesized by acylation of biphenyl with succinic anhydride under Friedel-Crafts conditions.
References
- Moore RA, Derry S, McQuay HJ (October 2009). "Single dose oral fenbufen for acute postoperative pain in adults". The Cochrane Database of Systematic Reviews. 2009 (4): CD007547. doi:10.1002/14651858.CD007547.pub2. PMC 4175557. PMID 19821427.
- Brogden RN (1986). "Non-steroidal anti-inflammatory analgesics other than salicylates". Drugs. 32 (Suppl 4): 27–45. doi:10.2165/00003495-198600324-00004. PMID 3552584. S2CID 25471102.
- "Deleted products 2010". Monthly Index of Medical Specialities (MIMS). Haymarket Media Group Ltd.
- Lewis JH, Stine JG (2013). "Nonsteroidal Antiinflammatory Drugs and Leukotriene Receptor Antagonists. Chapter 22". In Kaplowitz N, DeLeve LD (eds.). Drug-Induced Liver Disease (3rd ed.). Academic Press. ISBN 978-0-12-387818-2.
- "International listings for fenbufen". Drugs.com. Retrieved 25 June 2015.
- Castillo R, Suárez-Herrera M, Aparicio M, Hernández-Lui F, Hernández A (October 1995). "An Improved Synthesis of Fenbulen". Organic Preparations and Procedures International. 27 (5): 550–552. doi:10.1080/00304949509458497.
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |
This drug article relating to the musculoskeletal system is a stub. You can help Misplaced Pages by expanding it. |