 | |
| Names | |
|---|---|
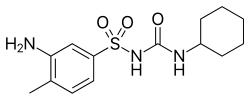
| Preferred IUPAC name 3-Amino-N-(cyclohexylcarbamoyl)-4-methylbenzene-1-sulfonamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.434 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H21N3O3S |
| Molar mass | 311.4 g/mol |
| Pharmacology | |
| ATC code | A10BB10 (WHO) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Metahexamide (INN) is an anti-diabetic drug from the group of sulfonylureas. It is long-acting and belongs to the first-generation cyclohexyl-containing sulfonylureas. It was first described in 1959.
References
- Moss JM, Delawter D (September 1959). "Metahexamide in diabetes therapy". Ann NY Acad Sci. 82 (2): 614–7. Bibcode:1959NYASA..82..614M. doi:10.1111/j.1749-6632.1959.tb44940.x. PMID 14424617.
| Ion channel modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium |
| ||||||||||||||||||||||||
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
| See also: Receptor/signaling modulators • Transient receptor potential channel modulators | |||||||||||||||||||||||||
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |