| This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (March 2024) (Learn how and when to remove this message) |
A metal-formaldehyde complex is a coordination complex in which a formaldehyde ligand has two bonds to the metal atom(s) (η-CH2O). This type of ligand has been reported in both monometallic and bimetallic complexes.
History
Metal-formaldehyde complexes have been reported for tungsten (W), osmium (Os), vanadium (V), rhenium (Re), zirconium (Zr), ruthenium (Ru), and niobium (Nb).
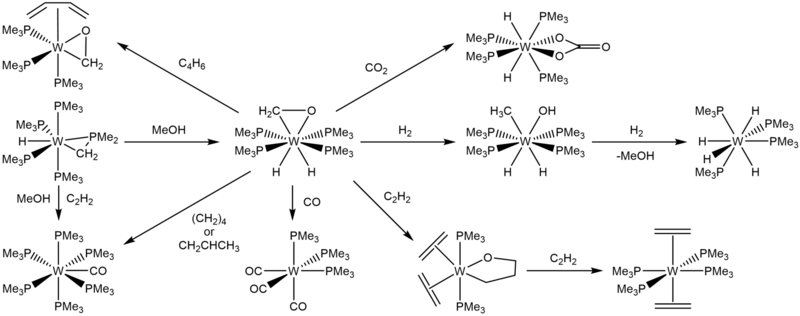
In 1984, Green and coworkers reported the yellow crystalline solid W(PMe3)4(η-CH2O)H2. It was the result of the addition of methanol to W(PMe3)4(η-CH2PMe2)H.
W(PMe3)4(η-CH2O)H2 can be hydrogenated to give W(PMe3)4(MeO)H3, and then further hydrogenated to reform methanol and generate W(PMe3)4H4. In 1986, Green and Parkin demonstrated further reactivities of W(PMe3)4(η-CH2O)H2. Upon addition of CO or CO2, W(PMe3)4(η-CH2O)H2 produces fac-W(PMe3)3(CO)3 and W(PMe3)4(κ-O2CO)H2, respectively, much like its precursor.
W(PMe3)4(η-CH2O)H2 also reacts with buta-1,3-diene to give W(PMe3)3(η-CH2O)(η-C4H6).
W(PMe3)4(η-CH2O)H2 can also be used as a route to further oxometallacycles by the addition of ethylene and rapid cooling to –80°C. The resultant green-colored crystals are composed of W(OCH2CH2CH2)(PMe3)2(η-C2H4)2, with either both ethylene ligands on the equatorial plane or the ethylene ligand cis- to the ligating oxygen in the axial direction. Further reaction with ethylene produces trans-W(PMe3)4(η-C2H4)2 and W(PMe3)4(CO)H2.
References
- Clark, G.R.; Headford, C.E.L.; Marsden, K.; Roper, W.R. (June 1982). "Synthesis, structure and reactions of a dihapto-formaldehyde complex, Os(η2-CH2O)(CO)2(PPh3)2". Journal of Organometallic Chemistry. 231 (4): 335–360. doi:10.1016/s0022-328x(00)81212-7. ISSN 0022-328X.
- Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (April 1982). "Metal-formaldehyde chemistry: coordination, disproportionation and Lewis acid-promoted transformation to oxymethylene derivatives". Journal of the American Chemical Society. 104 (7): 2019–2020. doi:10.1021/ja00371a038. ISSN 0002-7863.
- Buhro, William E.; Patton, Alan T.; Strouse, Charles E.; Gladysz, J. A.; McCormick, Fred B.; Etter, Margaret C. (February 1983). "Syntheses, chemical properties, and x-ray crystal structures of rhenium formaldehyde and thioformaldehyde complexes". Journal of the American Chemical Society. 105 (4): 1056–1058. doi:10.1021/ja00342a070. ISSN 0002-7863.
- Gambarotta, S.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. (March 1983). "Genesis, bonding mode and reaction with carbon monoxide of an oxymethylene unit bridging two metal atoms". Journal of the American Chemical Society. 105 (6): 1690–1691. doi:10.1021/ja00344a066. ISSN 0002-7863.
- Kropp, Kurt; Skibbe, Volker; Erker, Gerhard; Krueger, Carl (May 1983). "Fischer-Tropsch intermediates: tris[(.eta.2-formaldehyde)zirconocene] from the carbonylation of a zirconium hydride". Journal of the American Chemical Society. 105 (10): 3353–3354. doi:10.1021/ja00348a075. ISSN 0002-7863.
- Fachinetti, Giuseppe; Floriani, Carlo; Pucci, Sergio (1978-01-01). "Stoicheiometric reduction of CO and CO2 to methanol: evidence for carbon monoxide insertion into zirconium–hydrogen bond". Journal of the Chemical Society, Chemical Communications (6): 269–270. doi:10.1039/C39780000269. ISSN 0022-4936.
- Chaudret, Bruno N.; Cole-Hamilton, David J.; Nohr, Ronald S.; Wilkinson, Geoffrey (1977-01-01). "The reactions of chlorohydrido- and dichloro-tris(triphenylphosphine)ruthenium(II) with alkali hydroxides and alkoxides. Hydridohydroxobis(triphenylphosphine)ruthenium(II) monosolvates, their reactions and related compounds". Journal of the Chemical Society, Dalton Transactions (16): 1546–1557. doi:10.1039/DT9770001546. ISSN 1364-5447.
- Wolczanski, Peter T.; Threlkel, Richard S.; Bercaw, John E. (January 1979). "Reduction of coordinated carbon monoxide to "zirconoxy" carbenes with permethylzirconocene dihydride". Journal of the American Chemical Society. 101 (1): 218–220. doi:10.1021/ja00495a037. ISSN 0002-7863.
- Green, Malcolm L. H.; Parkin, Gerard; Moynihan, Kelly J.; Prout, Keith (1984-01-01). "Formation of an η2-formaldehyde compound from methanol and its hydrogenation giving methanol". Journal of the Chemical Society, Chemical Communications (22): 1540. doi:10.1039/C39840001540. ISSN 0022-4936.
- ^ Green, Malcolm L. H.; Parkin, Gerard (1986-01-01). "Ethylene insertion into the W–C bond of the η2-formaldehyde ligand system of W(PMe3)4(η2-CH2O)H2 giving the oxometallacyclopentane derivative W(OCH2CH2CH2)(PMe3)2(C2H4)2 and related studies". Journal of the Chemical Society, Chemical Communications (1): 90–91. doi:10.1039/C39860000090. ISSN 0022-4936.
| Coordination complexes | |
|---|---|
| H donors: | |
| B donors: | |
| C donors: | |
| Si donors: | |
| N donors: | |
| P donors: | |
| O donors: | |
| S donors: | |
| Halide donors: | |
This chemistry-related article is a stub. You can help Misplaced Pages by expanding it. |