| This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this article by introducing citations to additional sources. Find sources: "Methylcyclopropane" – news · newspapers · books · scholar · JSTOR (January 2024) |
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Methylcyclopropane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.934 | ||
| EC Number |
| ||
| MeSH | C105498 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H8 | ||
| Molar mass | 56.108 g·mol | ||
| Appearance | Colourless gas | ||
| Density | 0.6912 g/cm | ||
| Melting point | −177.3 °C (−287.1 °F; 95.8 K) | ||
| Boiling point | 0.7 °C (33.3 °F; 273.8 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
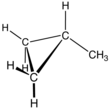
Methylcyclopropane is an organic compound with the structural formula C3H5CH3. This colorless gas is the monomethyl derivative of cyclopropane.
Reactions
Methylcyclopropane, like many other cyclopropanes, undergoes ring-opening reactions. Bond cleavage in certain reactions is also reported in conjunction with the use of methylenecyclopropane groups as protective groups for amines.
References
- ^ Lide, David. R, ed. (2009). CRC Handbook of Chemistry and Physics (89th ed.). CRC Press. ISBN 978-1-4200-6679-1.
| Hydrocarbons | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Unsaturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Aromatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||