 | |
| Names | |
|---|---|
| Preferred IUPAC name Trimethoxy(methyl)silane | |
| Other names MTM, Trimethoxymethylsilane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.350 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H12O3Si |
| Molar mass | 136.222 g·mol |
| Appearance | Colorless liquid |
| Density | 0.955 g/cm |
| Boiling point | 102–104 °C (216–219 °F; 375–377 K) |
| Solubility in water | hydrolysis |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
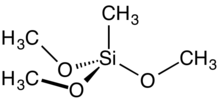
Methyltrimethoxysilane is an organosilicon compound with the formula CH3Si(OCH3)3. It is a colorless, free-flowing liquid. It is a crosslinker in the preparation of polysiloxane polymers.
Preparation, structure and reactivity
Methyltrimethoxysilane is usually prepared from methyltrichlorosilane and methanol:
- CH3SiCl3 + 3 CH3OH → CH3Si(OCH3)3 + 3 HCl
Alcoholysis of alkylchlorosilanes typically proceeds via an SN2 mechanism. Inversion of the configuration is favored during nucleophilic attack when displacing good leaving groups, such as chloride. In contrast, displacement of poor leaving groups, such as alkoxide, retention is favored.
Methyltrimethoxysilane is tetrahedral and is often described as sp hybridized. It has idealized C3v point symmetry.
Hydrolysis of MTM proceeds both under acidic and basic conditions. Under acid conditions, rates of successive hydrolyses for methyltrimethoxysilane decreases with each step. Under basic condition the opposite is true.
See also
References
- ^ Brinker, C.J. (1988). "Hydrolysis and condensation of silicates: Effects on structure" (PDF). Journal of Non-Crystalline Solids. 100: 31. Bibcode:1988JNCS..100...31B. doi:10.1016/0022-3093(88)90005-1.
- Kuroda, Kazuyuki; Shimojima, Atsushi; Kawahara, Kazufumi; Wakabayashi, Ryutaro; Tamura, Yasuhiro; Asakura, Yusuke; Kitahara, Masaki (2014). "Utilization of Alkoxysilyl Groups for the Creation of Structurally Controlled Siloxane-Based Nanomaterials". Chemistry of Materials. 26: 211. doi:10.1021/cm4023387.
- Stephan, Pawlenko (1986). Organosilicon Chemistry. Berlin: Walter de Gruyter. ISBN 9780899252025.
- Colvin, Ernest W. (1981). Silicon in Organic Synthesis. Butterworth and Co Ltd. doi:10.1016/c2013-0-04155-2. ISBN 978-0-408-10831-7.