 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
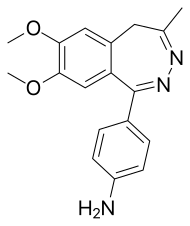
| Formula | C18H19N3O2 |
| Molar mass | 309.369 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Nerisopam (GYKI-52322, EGIS-6775) is a drug which is a 2,3-benzodiazepine derivative, related to tofisopam. It has potent anxiolytic and neuroleptic effects in animal studies.
See also
References
- Horváth K, Andrási F, Berzsenyi P, Pátfalusi M, Patthy M, Szabó G, Sebestyén L, Bagdy E, Körösi J, Botka P (August 1989). "A new psychoactive 5H-2,3-benzodiazepine with a unique spectrum of activity". Arzneimittel-Forschung. 39 (8): 894–9. PMID 2573361.
- Horváth K, Andrási F, Botka P, Hámori T (1992). "Anxiolytic profile of girisopam and GYKI 52,322 (EGIS 6775). Comparison with chlordiazepoxide and buspirone". Acta Physiologica Hungarica. 79 (2): 153–61. PMID 1363928.
- Palkovits M, Baffi JS, Berzsenyi P, Horváth EJ (July 1997). "Anxiolytic homophthalazines increase Fos-like immunoreactivity in selected brain areas of the rat". European Journal of Pharmacology. 331 (1): 53–63. doi:10.1016/s0014-2999(97)01008-x. PMID 9274930.
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |