 | |
| Names | |
|---|---|
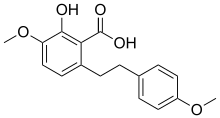
| IUPAC name 2-hydroxy-4-methoxy-6-benzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.069.726 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H18O5 |
| Molar mass | 302.326 g·mol |
| Melting point | 149 to 150 °C (300 to 302 °F; 422 to 423 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Notholaenic acid is a dihydrostilbenoid found in the farina of some ferns of the genus Notholaena. It has been shown to have anti-HSV-1 (Herpes simplex virus 1) activity at high concentrations in vitro. It was artificially synthesized, starting from 3-benzyloxy-5-methoxybenzyl alcohol, in 1985.
References
- Wollenweber, Eckhard; Favre-Bonvin, Jean (1979). "Novel dihydrostilbene from fronds of Notholaena dealbata and Notholaena limitanea". Phytochemistry. 18 (7): 1243–1244. doi:10.1016/0031-9422(79)80153-3.
- Rinehart, Kenneth L.; Tom G. Holt; Nancy L. Fregeau; Paul A. Keifer; George Robert Wilson; Thomas J. Perun Jr; Ryuichi Sakai; Anthony G. Thompson; Justin G. Stroh; Lois S. Shield; David S. Seigler; Li H. Li; David G. Martin; Cornelis J. P. Grimmelikhuijzen; Gerd Gäde (July–August 1990). "Bioactive Compounds from Aquatic and Terrestrial Sources". Journal of Natural Products. 53 (4): 771–792. doi:10.1021/np50070a001. PMID 2095373.
- El-Feraly, Farouk S.; Cheatham, Steve F.; McChesney, James D. (1985). "Total Synthesis of Notholaenic Acid". Journal of Natural Products. 48 (2): 293–298. doi:10.1021/np50038a015.
| Dihydrostilbenoids and their glycosides | |
|---|---|
| Dihydrostilbenoids |
|
| Oligomeric forms |
|
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |