This page provides supplementary chemical data on o-Xylene.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as eChemPortal, and follow its directions. MSDS is available from MATHESON TRI-GAS, INC. in the SDSdata.org database.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.5058 at 20°C |
| Abbe number | ? |
| Dielectric constant, εr | 2.568 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension | 30.10 dyn/cm at 20°C |
| Viscosity | 1.1049 mPa·s at 0°C 0.8102 mPa·s at 20°C 0.6270 mPa·s at 40°C 0.4168 mPa·s at 80°C 0.2626 mPa·s at 140°C |
| Solubility | 0.171 g/L at 25°C 0.21 g/L at 45°C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 247.8 K (−25.3 °C), ? Pa |
| Critical point | 631 K (358 °C), 3700 kPa |
| Std enthalpy change of fusion, ΔfusH |
13.6 kJ/mol |
| Std entropy change of fusion, ΔfusS |
54.87 J/(mol·K) at −25.3 |
| Std enthalpy change of vaporization, ΔvapH |
36.24 kJ/mol at 144.5°C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfHsolid |
? kJ/mol |
| Standard molar entropy, Ssolid |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfHliquid |
−24.4 kJ/mol |
| Standard molar entropy, Sliquid |
247 J/(mol K) |
| Enthalpy of combustion, ΔcH | −4552 kJ/mol |
| Heat capacity, cp | 187.0 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfHgas |
19.0 kJ/mol |
| Standard molar entropy, Sgas |
353.6 J/(mol K) |
| Heat capacity, cp | 132.5 J/(mol K) at 25°C |
| van der Waals' constants | a = 3038 L kPa/mol b = 0.1755 liter per mole |
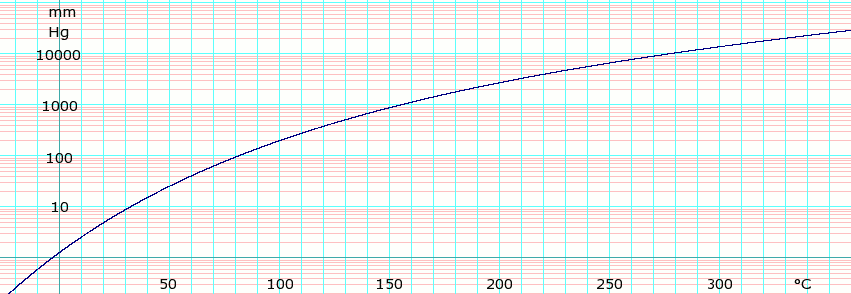
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | −3.8 | 32.1 | 59.5 | 81.3 | 121.7 | 144.4 | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Distillation data
See also:
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
- Merck Index of Chemicals and Drugs, 9th ed. monograph 9743
- CRC Handbook of Chemistry and Physics, 44th ed. pp 2611–2620
- CRC Handbook of Chemistry and Physics, 44th ed. pp 2244–2248
- Lange's Handbook of Chemistry, 10th ed. pp 1669–1674
- CRC Handbook of Chemistry and Physics, 85th ed. p8-111
- Lange's Handbook of Chemistry, 10th ed, pp 1522–1524
- "Pure Component Properties" (Queriable Database). Chemical Engineering Research Information Center. Retrieved 27 May 2007.
- ^ "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 27 May 2007.
- Linstrom, Peter (1997). "NIST Standard Reference Database". National Institute of Standards and Technology. doi:10.18434/T4D303.
{{cite journal}}: Cite journal requires|journal=(help)
Category:

 obtained from CHERIC
obtained from CHERIC