 | |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.276 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | I2Pd |
| Molar mass | 360.229 g/mol |
| Appearance | Black crystals |
| Density | 6,003 g/cm |
| Melting point | 350 °C (decomposes) |
| Solubility in water | Insoluble in water |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Related compounds | |
| Other anions | Palladium(II) fluoride Palladium(II) chloride Palladium(II) bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Palladium(II) iodide is an inorganic compound of palladium and iodine. It is commercially available, though less common than palladium(II) chloride, the usual entry point to palladium chemistry. Three polymorphs are known.
Preparation
Palladium(II) iodide can be obtained by treating a dilute solution of palladium in nitric acid with sodium iodide at 80 °C.
The high-temperature polymorph α-palladium(II) iodide can be produced by reaction of the elements at temperature above 600 °C. The γ-modification is produced as an almost amorphous powder by addition of iodide salts to aqueous H2PdCl4 solution . When heated in dilute hydrogen iodide solution, this polymorph transforms into the β phase at around 140 °C.
Reactions and uses
Palladium(II) iodide is insoluble in water. It reacts with iodide giving PdI4 anion:
- PdI2 + 2I → PdI2−4
It finds use as a catalyst.
Historically, the quantity of palladium in a solution may be determined gravimetrically by precipitation as palladium(II) iodide.
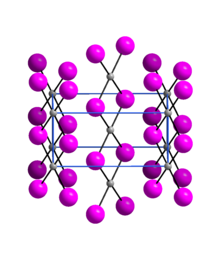
Crystallography
Palladium(II) iodide is an almost X-ray amorphous black powder. The α-modification has an orthorhombic crystal structure with the space group Pnmn(space group no. 58, position 5).
References
- "C&L Inventory". echa.europa.eu. Retrieved 13 December 2021.
- ^ Handbuch der präparativen anorganischen Chemie. 3 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1981. ISBN 978-3-432-87823-2.
- Brendel, Kristin; Thiele, Gerhard (2001). "Binäre und Ternäre Verbindungen der Platinmetalle Palladium und Rhodium mit Tellur und Halogenen. Präparationen und strukturelle Charakterisierung".
{{cite journal}}: Cite journal requires|journal=(help) - Gabriele, Bartolo; Salerno, Giuseppe (2006), "Palladium(II) Iodide", Encyclopedia of Reagents for Organic Synthesis, American Cancer Society, doi:10.1002/047084289x.rn00658, ISBN 978-0-470-84289-8, retrieved 2021-03-26
- Beamish, F. E.; Dale, J. (1938). "Determination of Palladium by Means of Potassium Iodide". Industrial & Engineering Chemistry Analytical Edition. 10 (12): 697. doi:10.1021/ac50128a015.
- Ans, Jean d'; Lax, Ellen (1998). Taschenbuch für Chemiker und Physiker (in German). Springer. ISBN 978-3-540-60035-0.
| Palladium compounds | |||
|---|---|---|---|
| Pd(0) |
| ||
| Pd(II) |
| ||
| Pd(II,IV) | |||
| Pd(IV) | |||
| Pd(VI) | |||
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||