 | |
| Names | |
|---|---|
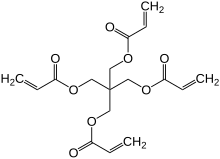
| Preferred IUPAC name 2,2-Bis{methyl}propane-1,3-diyl di(prop-2-enoate) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.023.313 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H20O8 |
| Molar mass | 352.339 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H317, H319 |
| Precautionary statements | P261, P264, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362, P363, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Pentaerythritol tetraacrylate (PETA, sometimes PETTA, PETRA) is an organic compound. It is a tetrafunctional acrylate ester used as a monomer in the manufacture of polymers. As it is a polymerizable acrylate monomer, it is nearly always supplied with an added polymerisation inhibitor, such as MEHQ (monomethyl ether hydroquinone).
Uses
PETA is part of a family of acrylates used in epoxy resin chemistry and ultraviolet cure of coatings. Similar monomers used are 1,6-hexanediol diacrylate and trimethylol propane triacrylate. It is a derivative of pentaerythritol One of the key uses of the material is in polymeric synthesis where it can form micelles and block copolymers. The molecule's acrylate group functionality enables the molecule to do the Michael reaction with amines. It is therefore sometimes used in epoxy chemistry enabling a large reduction in cure time. As the molecule has 4 functional acrylate groups it confers high cross-link density. Ethoxylation maybe used to produce ethoxylated versions which find use in electron beam curing. The material also has pharmaceutical uses
See also
References
- "Pentaerythritol tetraacrylate". webbook.nist.gov. Archived from the original on 2021-02-08. Retrieved 2020-03-17.
- Marrian, S. F. (1948-08-01). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. ISSN 0009-2665. PMID 18876970. Archived from the original on 2021-02-08. Retrieved 2020-03-17.
- petrov, P (2008). "Wormlike morphology formation and stabilization of Pluronic P123 micelles by solubilization of pentaerythritol tetraacrylate". The Journal of Physical Chemistry. B 112(30) (30): 8879–8883. doi:10.1021/jp8008767. PMID 18598071.
- Petrov, Petar; Bozukov, Metodi; Burkhardt, Markus; Muthukrishnan, Sharmila; Müller, Axel H. E.; Tsvetanov, Christo B. (2006-05-31). "Stabilization of polymeric micelles with a mixed poly(ethylene oxide)/poly(2-hydroxyethyl methacrylate) shell by formation of poly(pentaerythritol tetraacrylate) nanonetworks within the micelles". Journal of Materials Chemistry. 16 (22): 2192–2199. doi:10.1039/B517028A. ISSN 1364-5501. Archived from the original on 2020-03-17. Retrieved 2020-03-17.
- "Epoxy Polyacrylate Resins". www.hexion.com. Archived from the original on 2020-02-13. Retrieved 2020-03-17.
- Chowdhury, Rajesh (2007). "Electron-beam-induced crosslinking of natural rubber/acrylonitrile–butadiene rubber latex blends in the presence of ethoxylated pentaerythritol tetraacrylate used as a crosslinking promoter". Journal of Applied Polymer Science. 103 (2): 1206–1214. doi:10.1002/app.25383. ISSN 1097-4628. Archived from the original on 2020-03-17. Retrieved 2020-03-17.
- Wong, Rachel Shet Hui; Ashton, Mark; Dodou, Kalliopi (2016-10-01). "Analysis of residual crosslinking agent content in UV cross-linked poly(ethylene oxide) hydrogels for dermatological application by gas chromatography". Journal of Pharmaceutical Analysis. 6 (5): 307–312. doi:10.1016/j.jpha.2016.04.004. ISSN 2095-1779. PMC 5762621. PMID 29403997.