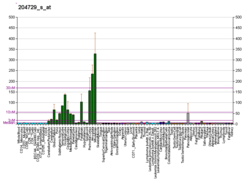
Syntaxin-1A is a protein that in humans is encoded by the STX1A gene.
Function
Synaptic vesicles store neurotransmitters that are released during calcium-regulated exocytosis. The specificity of neurotransmitter release requires the localization of both synaptic vesicles and calcium channels to the presynaptic active zone. Syntaxins function in this vesicle fusion process.
Syntaxin-1A is a member of the syntaxin superfamily. Syntaxins are nervous system-specific proteins implicated in the docking of synaptic vesicles with the presynaptic plasma membrane. Syntaxins possess a single C-terminal transmembrane domain, a SNARE domain (known as H3), and an N-terminal regulatory domain (Habc). Syntaxins bind synaptotagmin in a calcium-dependent fashion and interact with voltage dependent calcium and potassium channels via the C-terminal H3 domain. Syntaxin-1A is a key protein in ion channel regulation and synaptic exocytosis.
Clinical significance
Syntaxins serve as a substrate for botulinum neurotoxin type C, a metalloprotease that blocks exocytosis and has high affinity for a molecular complex that includes the alpha-latrotoxin receptor which produces explosive exocytosis.
The expression level of STX1A is directly correlated with intelligence in Williams syndrome.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.
[[File:

- The interactive pathway map can be edited at WikiPathways: "NicotineDopaminergic_WP1602".
Interactions
STX1A has been shown to interact with:
- CPLX1,
- CFTR,
- NAPA,
- RNF40,
- SCNN1G,
- SLC6A1,
- SNAP-25,
- SNAP23,
- STXBP1,
- STXBP5,
- SYT1
- UNC13B,
- VAMP2, and
- VAMP8.
See also
References
- ^ GRCh38: Ensembl release 89: ENSG00000106089 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000007207 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Bennett MK, Calakos N, Scheller RH (July 1992). "Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones". Science. 257 (5067): 255–9. Bibcode:1992Sci...257..255B. doi:10.1126/science.1321498. PMID 1321498.
- "Entrez Gene: STX1A syntaxin 1A (brain)".
- Zhang R, Maksymowych AB, Simpson LL (July 1995). "Cloning and sequence analysis of a cDNA encoding human syntaxin 1A, a polypeptide essential for exocytosis". Gene. 159 (2): 293–4. doi:10.1016/0378-1119(95)00152-V. PMID 7622072.
- Gao MC, Bellugi U, Dai L, Mills DL, Sobel EM, Lange K, Korenberg JR (2010). Toland AE (ed.). "Intelligence in Williams Syndrome is related to STX1A, which encodes a component of the presynaptic SNARE complex". PLOS ONE. 5 (4): e10292. Bibcode:2010PLoSO...510292G. doi:10.1371/journal.pone.0010292. PMC 2858212. PMID 20422020.
- Brian Maffly (2010-04-22). "U. scientist links one gene to intelligence". The Salt Lake Tribune. Archived from the original on 2010-04-25.
- ^ Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J (August 1999). "A conformational switch in syntaxin during exocytosis: role of munc18". The EMBO Journal. 18 (16): 4372–82. doi:10.1093/emboj/18.16.4372. PMC 1171512. PMID 10449403.
- ^ Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, Rizo J (January 2002). "Three-dimensional structure of the complexin/SNARE complex". Neuron. 33 (3): 397–409. doi:10.1016/S0896-6273(02)00583-4. PMID 11832227. S2CID 17878965.
- Hu K, Carroll J, Rickman C, Davletov B (November 2002). "Action of complexin on SNARE complex". The Journal of Biological Chemistry. 277 (44): 41652–6. doi:10.1074/jbc.M205044200. PMID 12200427.
- Naren AP, Nelson DJ, Xie W, Jovov B, Pevsner J, Bennett MK, Benos DJ, Quick MW, Kirk KL (November 1997). "Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms". Nature. 390 (6657): 302–5. Bibcode:1997Natur.390..302N. doi:10.1038/36882. PMID 9384384. S2CID 4395005.
- Cormet-Boyaka E, Di A, Chang SY, Naren AP, Tousson A, Nelson DJ, Kirk KL (September 2002). "CFTR chloride channels are regulated by a SNAP-23/syntaxin 1A complex". Proceedings of the National Academy of Sciences of the United States of America. 99 (19): 12477–82. Bibcode:2002PNAS...9912477C. doi:10.1073/pnas.192203899. PMC 129470. PMID 12209004.
- ^ McMahon HT, Missler M, Li C, Südhof TC (October 1995). "Complexins: cytosolic proteins that regulate SNAP receptor function". Cell. 83 (1): 111–9. doi:10.1016/0092-8674(95)90239-2. PMID 7553862. S2CID 675343.
- Hanson PI, Otto H, Barton N, Jahn R (July 1995). "The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin". The Journal of Biological Chemistry. 270 (28): 16955–61. doi:10.1074/jbc.270.28.16955. PMID 7622514.
- Chin LS, Vavalle JP, Li L (September 2002). "Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation". The Journal of Biological Chemistry. 277 (38): 35071–9. doi:10.1074/jbc.M203300200. PMID 12121982.
- Berdiev BK, Jovov B, Tucker WC, Naren AP, Fuller CM, Chapman ER, Benos DJ (June 2004). "ENaC subunit-subunit interactions and inhibition by syntaxin 1A". American Journal of Physiology. Renal Physiology. 286 (6): F1100–6. doi:10.1152/ajprenal.00344.2003. PMID 14996668. S2CID 18384316.
- Beckman ML, Bernstein EM, Quick MW (August 1998). "Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A". The Journal of Neuroscience. 18 (16): 6103–12. doi:10.1523/JNEUROSCI.18-16-06103.1998. PMC 6793212. PMID 9698305.
- Quick MW (April 2002). "Substrates regulate gamma-aminobutyric acid transporters in a syntaxin 1A-dependent manner". Proceedings of the National Academy of Sciences of the United States of America. 99 (8): 5686–91. Bibcode:2002PNAS...99.5686Q. doi:10.1073/pnas.082712899. PMC 122832. PMID 11960023.
- Deken SL, Beckman ML, Boos L, Quick MW (October 2000). "Transport rates of GABA transporters: regulation by the N-terminal domain and syntaxin 1A". Nature Neuroscience. 3 (10): 998–1003. doi:10.1038/79939. PMID 11017172. S2CID 11312913.
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- ^ Hata Y, Südhof TC (June 1995). "A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic". The Journal of Biological Chemistry. 270 (22): 13022–8. doi:10.1074/jbc.270.22.13022. PMID 7768895.
- Gonelle-Gispert C, Molinete M, Halban PA, Sadoul K (September 2000). "Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells". Journal of Cell Science. 113 ( Pt 18) (18): 3197–205. doi:10.1242/jcs.113.18.3197. PMID 10954418.
- Ilardi JM, Mochida S, Sheng ZH (February 1999). "Snapin: a SNARE-associated protein implicated in synaptic transmission". Nature Neuroscience. 2 (2): 119–24. doi:10.1038/5673. PMID 10195194. S2CID 25524692.
- Li Y, Chin LS, Weigel C, Li L (November 2001). "Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis". The Journal of Biological Chemistry. 276 (44): 40824–33. doi:10.1074/jbc.M106141200. PMID 11524423.
- ^ Ravichandran V, Chawla A, Roche PA (June 1996). "Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues". The Journal of Biological Chemistry. 271 (23): 13300–3. doi:10.1074/jbc.271.23.13300. PMID 8663154.
- Chapman ER, An S, Barton N, Jahn R (November 1994). "SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils". The Journal of Biological Chemistry. 269 (44): 27427–32. doi:10.1016/S0021-9258(18)47003-2. PMID 7961655.
- ^ Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH (December 1998). "Three novel proteins of the syntaxin/SNAP-25 family". The Journal of Biological Chemistry. 273 (51): 34171–9. doi:10.1074/jbc.273.51.34171. PMID 9852078.
- Imai A, Nashida T, Yoshie S, Shimomura H (August 2003). "Intracellular localisation of SNARE proteins in rat parotid acinar cells: SNARE complexes on the apical plasma membrane". Archives of Oral Biology. 48 (8): 597–604. doi:10.1016/S0003-9969(03)00116-X. PMID 12828989.
- Li G, Alexander EA, Schwartz JH (May 2003). "Syntaxin isoform specificity in the regulation of renal H+-ATPase exocytosis". The Journal of Biological Chemistry. 278 (22): 19791–7. doi:10.1074/jbc.M212250200. PMID 12651853.
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M (May 1997). "Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c". Biochemical and Biophysical Research Communications. 234 (1): 257–62. doi:10.1006/bbrc.1997.6560. hdl:20.500.14094/D2002245. PMID 9168999.
- Bhaskar K, Shareef MM, Sharma VM, Shetty AP, Ramamohan Y, Pant HC, Raju TR, Shetty KT (January 2004). "Co-purification and localization of Munc18-1 (p67) and Cdk5 with neuronal cytoskeletal proteins". Neurochemistry International. 44 (1): 35–44. doi:10.1016/S0197-0186(03)00099-8. PMID 12963086. S2CID 23783141.
- ^ Pérez-Brangulí F, Muhaisen A, Blasi J (June 2002). "Munc 18a binding to syntaxin 1A and 1B isoforms defines its localization at the plasma membrane and blocks SNARE assembly in a three-hybrid system assay". Molecular and Cellular Neurosciences. 20 (2): 169–80. doi:10.1006/mcne.2002.1122. PMID 12093152. S2CID 23927545.
- Widberg CH, Bryant NJ, Girotti M, Rea S, James DE (September 2003). "Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation". The Journal of Biological Chemistry. 278 (37): 35093–101. doi:10.1074/jbc.M304261200. PMID 12832401.
- Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH, Takai Y (May 1998). "Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process". Neuron. 20 (5): 905–15. doi:10.1016/S0896-6273(00)80472-9. PMID 9620695. S2CID 12597505.
- Shao X, Li C, Fernandez I, Zhang X, Südhof TC, Rizo J (January 1997). "Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch". Neuron. 18 (1): 133–42. doi:10.1016/S0896-6273(01)80052-0. PMID 9010211. S2CID 17947552.
- Thomas DM, Ferguson GD, Herschman HR, Elferink LA (July 1999). "Functional and biochemical analysis of the C2 domains of synaptotagmin IV". Molecular Biology of the Cell. 10 (7): 2285–95. doi:10.1091/mbc.10.7.2285. PMC 25443. PMID 10397765.
- Betz A, Okamoto M, Benseler F, Brose N (January 1997). "Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin". The Journal of Biological Chemistry. 272 (4): 2520–6. doi:10.1074/jbc.272.4.2520. PMID 8999968.
- Margittai M, Otto H, Jahn R (March 1999). "A stable interaction between syntaxin 1a and synaptobrevin 2 mediated by their transmembrane domains". FEBS Letters. 446 (1): 40–4. doi:10.1016/S0014-5793(99)00028-9. PMID 10100611. S2CID 9115709.
- Hao JC, Salem N, Peng XR, Kelly RB, Bennett MK (March 1997). "Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes". The Journal of Neuroscience. 17 (5): 1596–603. doi:10.1523/JNEUROSCI.17-05-01596.1997. PMC 6573372. PMID 9030619.
- Nagamatsu S, Nakamichi Y, Watanabe T, Matsushima S, Yamaguchi S, Ni J, Itagaki E, Ishida H (January 2001). "Localization of cellubrevin-related peptide, endobrevin, in the early endosome in pancreatic beta cells and its physiological function in exo-endocytosis of secretory granules". Journal of Cell Science. 114 (Pt 1): 219–227. doi:10.1242/jcs.114.1.219. PMID 11112705.
Further reading
- Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA (2002). "Role of snare proteins in CFTR and ENaC trafficking". Pflügers Archiv. 443 (Suppl 1): S65–9. doi:10.1007/s004240100647. PMID 11845306. S2CID 21790638.
- Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE (May 1992). "A multisubunit particle implicated in membrane fusion". The Journal of Cell Biology. 117 (3): 531–8. doi:10.1083/jcb.117.3.531. PMC 2289450. PMID 1315316.
- McMahon HT, Missler M, Li C, Südhof TC (October 1995). "Complexins: cytosolic proteins that regulate SNAP receptor function". Cell. 83 (1): 111–9. doi:10.1016/0092-8674(95)90239-2. PMID 7553862. S2CID 675343.
- Zhang R, Maksymowych AB, Simpson LL (July 1995). "Cloning and sequence analysis of a cDNA encoding human syntaxin 1A, a polypeptide essential for exocytosis". Gene. 159 (2): 293–4. doi:10.1016/0378-1119(95)00152-V. PMID 7622072.
- Hanson PI, Otto H, Barton N, Jahn R (July 1995). "The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin". The Journal of Biological Chemistry. 270 (28): 16955–61. doi:10.1074/jbc.270.28.16955. PMID 7622514.
- Hata Y, Südhof TC (June 1995). "A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic". The Journal of Biological Chemistry. 270 (22): 13022–8. doi:10.1074/jbc.270.22.13022. PMID 7768895.
- Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Südhof TC (June 1995). "Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins". Nature. 375 (6532): 594–9. Bibcode:1995Natur.375..594L. doi:10.1038/375594a0. PMID 7791877. S2CID 4265549.
- Pevsner J, Hsu SC, Scheller RH (February 1994). "n-Sec1: a neural-specific syntaxin-binding protein". Proceedings of the National Academy of Sciences of the United States of America. 91 (4): 1445–9. Bibcode:1994PNAS...91.1445P. doi:10.1073/pnas.91.4.1445. PMC 43176. PMID 8108429.
- Betz A, Okamoto M, Benseler F, Brose N (January 1997). "Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin". The Journal of Biological Chemistry. 272 (4): 2520–6. doi:10.1074/jbc.272.4.2520. PMID 8999968.
- Jagadish MN, Tellam JT, Macaulay SL, Gough KH, James DE, Ward CW (January 1997). "Novel isoform of syntaxin 1 is expressed in mammalian cells". The Biochemical Journal. 321 ( Pt 1) (Pt 1): 151–6. doi:10.1042/bj3210151. PMC 1218049. PMID 9003414.
- Shao X, Li C, Fernandez I, Zhang X, Südhof TC, Rizo J (January 1997). "Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch". Neuron. 18 (1): 133–42. doi:10.1016/S0896-6273(01)80052-0. PMID 9010211. S2CID 17947552.
- Hao JC, Salem N, Peng XR, Kelly RB, Bennett MK (March 1997). "Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes". The Journal of Neuroscience. 17 (5): 1596–603. doi:10.1523/JNEUROSCI.17-05-01596.1997. PMC 6573372. PMID 9030619.
- Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE (March 1997). "Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4". The Journal of Biological Chemistry. 272 (10): 6179–86. doi:10.1074/jbc.272.10.6179. PMID 9045631.
- Coffield JA, Bakry N, Zhang RD, Carlson J, Gomella LG, Simpson LL (March 1997). "In vitro characterization of botulinum toxin types A, C and D action on human tissues: combined electrophysiologic, pharmacologic and molecular biologic approaches". The Journal of Pharmacology and Experimental Therapeutics. 280 (3): 1489–98. PMID 9067339.
- Nakayama T, Fujiwara T, Miyazawa A, Asakawa S, Shimizu N, Shimizu Y, Mikoshiba K, Akagawa K (May 1997). "Mapping of the human HPC-1/syntaxin 1A gene (STX1A) to chromosome 7 band q11.2". Genomics. 42 (1): 173–6. doi:10.1006/geno.1997.4650. PMID 9177791.
- Osborne LR, Soder S, Shi XM, Pober B, Costa T, Scherer SW, Tsui LC (August 1997). "Hemizygous deletion of the syntaxin 1A gene in individuals with Williams syndrome". American Journal of Human Genetics. 61 (2): 449–52. doi:10.1086/514850. PMC 1715888. PMID 9311751.
- Naren AP, Nelson DJ, Xie W, Jovov B, Pevsner J, Bennett MK, Benos DJ, Quick MW, Kirk KL (November 1997). "Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms". Nature. 390 (6657): 302–5. Bibcode:1997Natur.390..302N. doi:10.1038/36882. PMID 9384384. S2CID 4395005.
- Okamoto M, Südhof TC (December 1997). "Mints, Munc18-interacting proteins in synaptic vesicle exocytosis". The Journal of Biological Chemistry. 272 (50): 31459–64. doi:10.1074/jbc.272.50.31459. PMID 9395480.
- Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL (February 1998). "Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings". The Journal of Biological Chemistry. 273 (9): 4957–66. doi:10.1074/jbc.273.9.4957. PMID 9478941.
- Vinogradova TI (January 1977). "". Problemy Tuberkuleza (in Russian) (1): 19–22. PMID 834751.
- Burgess RW, Deitcher DL, Schwarz TL (August 1997). "The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos". The Journal of Cell Biology. 138 (4): 861–75. doi:10.1083/jcb.138.4.861. PMC 2138053. PMID 9265652.
- Littleton JT, Chapman ER, Kreber R, Garment MB, Carlson SD, Ganetzky B (August 1998). "Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly". Neuron. 21 (2): 401–13. doi:10.1016/S0896-6273(00)80549-8. PMID 9728921. S2CID 15119362.
- Lagow RD, Bao H, Cohen EN, Daniels RW, Zuzek A, Williams WH, Macleod GT, Sutton RB, Zhang B (April 2007). "Modification of a hydrophobic layer by a point mutation in syntaxin 1A regulates the rate of synaptic vesicle fusion". PLOS Biology. 5 (4): e72. doi:10.1371/journal.pbio.0050072. PMC 1808484. PMID 17341138.
External links
- Overview of all the structural information available in the PDB for UniProt: P32851 (Rat Syntaxin-1A) at the PDBe-KB.
| PDB gallery | |
|---|---|
|
| Membrane protein: vesicular transport proteins (TC 1F) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synaptic vesicle |
| ||||||||||||||||||||||||||
| COPI | |||||||||||||||||||||||||||
| COPII | |||||||||||||||||||||||||||
| RME/Clathrin | |||||||||||||||||||||||||||
| Caveolae | |||||||||||||||||||||||||||
| Other/ungrouped |
| ||||||||||||||||||||||||||
| See also vesicular transport protein disorders | |||||||||||||||||||||||||||
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
Category: