 | |
| Names | |
|---|---|
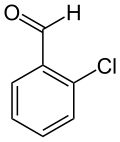
| Preferred IUPAC name 2-Chlorobenzaldehyde | |
| Other names o-Chlorobenzaldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.779 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3265 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H5ClO |
| Molar mass | 140.57 g·mol |
| Appearance | Clear colourless to pale yellow oily liquid |
| Density | 1.25 |
| Melting point | 9–12 °C (48–54 °F; 282–285 K) |
| Boiling point | 209–215 °C (408–419 °F; 482–488 K) |
| Solubility in water | Insoluble |
| Solubility | Slightly soluble in Acetonitrile, sparingly soluble in DMSO |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H290, H302, H314, H317 |
| Precautionary statements | P234, P260, P261, P264, P270, P272, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P333+P313, P363, P390, P404, P405, P501 |
| Flash point | 87 °C (189 °F; 360 K) |
| Autoignition temperature |
385 °C (725 °F; 658 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2-Chlorobenzaldehyde (o-chlorobenzaldehyde) is an organic compound with the formula ClC5H4CHO. It is one of three isomeric monochlorinated benzaldehyde. 3-Chlorobenzaldehyde and 4-chlorobenzaldehyde are the other isomers. Whereas benzaldehyde is prone to autoxidation, the 2-chloro derivatives are more air-stable.
Synthesis and uses
It is produced by hydrolysis of 2-chlorobenzal chloride:
- ClC5H4CHCl2 + H2O → ClC5H4CHO + 2 HCl
2-Chlorobenzaldehyde is used in production of CS gas. It reacts with malononitrile to form CS.
References
- Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3-527-30385-4.
- "Synthesis of ortho-chlorobenzalmalononitrile".
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |