 | |
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Caesium iodide | |
| Other names Cesium iodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.223 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CsI |
| Molar mass | 259.809 g/mol |
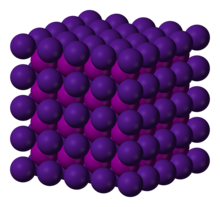
| Appearance | white crystalline solid |
| Density | 4.51 g/cm |
| Melting point | 632 °C (1,170 °F; 905 K) |
| Boiling point | 1,280 °C (2,340 °F; 1,550 K) |
| Solubility in water | 848 g/L (25 °C) |
| Magnetic susceptibility (χ) | -82.6·10 cm/mol |
| Refractive index (nD) | 1.9790 (0.3 µm) 1.7873 (0.59 µm) 1.7694 (0.75 µm) 1.7576 (1 µm) 1.7428 (5 µm) 1.7280 (20 µm) |
| Structure | |
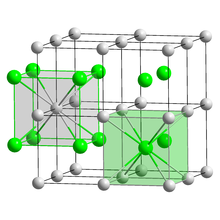
| Crystal structure | CsCl, cP2 |
| Space group | Pm3m, No. 221 |
| Lattice constant | a = 0.4503 nm |
| Lattice volume (V) | 0.0913 nm |
| Formula units (Z) | 1 |
| Coordination geometry | Cubic (Cs) Cubic (I) |
| Thermochemistry | |
| Heat capacity (C) | 52.8 J/mol·K |
| Std molar entropy (S298) |
123.1 J/mol·K |
| Std enthalpy of formation (ΔfH298) |
−346.6 kJ/mol |
| Gibbs free energy (ΔfG) | -340.6 kJ/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Warning |
| Hazard statements | H315, H317, H319, H335 |
| Precautionary statements | P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 2386 mg/kg (oral, rat) |
| Related compounds | |
| Other anions | Caesium fluoride Caesium chloride Caesium bromide Caesium astatide |
| Other cations | Lithium iodide Sodium iodide Potassium iodide Rubidium iodide Francium iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.
Synthesis and structure
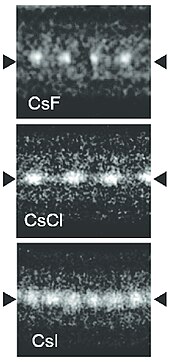
Bulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates.
Caesium iodide atomic chains can be grown inside double-wall carbon nanotubes. In such chains I atoms appear brighter than Cs atoms in electron micrographs despite having a smaller mass. This difference was explained by the charge difference between Cs atoms (positive), inner nanotube walls (negative) and I atoms (negative). As a result, Cs atoms are attracted to the walls and vibrate more strongly than I atoms, which are pushed toward the nanotube axis.
Properties
| Т (°C) | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (wt%) | 30.9 | 37.2 | 43.2 | 45.9 | 48.6 | 53.3 | 57.3 | 60.7 | 63.6 | 65.9 | 67.7 | 69.2 |
Applications
An important application of caesium iodide crystals, which are scintillators, is electromagnetic calorimetry in experimental particle physics. Pure CsI is a fast and dense scintillating material with relatively low light yield that increases significantly with cooling, and a fairly small Molière radius is 3.5 cm. It exhibits two main emission components: one in the near ultraviolet region at the wavelength of 310 nm and one at 460 nm. The drawbacks of CsI are a high temperature gradient and a slight hygroscopicity.
Caesium iodide is used as a beamsplitter in Fourier transform infrared (FTIR) spectrometers. It has a wider transmission range than the more common potassium bromide beamsplitters, working range into the far infrared. However, optical-quality CsI crystals are very soft and hard to cleave or polish. They should also be coated (typically with germanium) and stored in a desiccator, to minimize interaction with atmospheric water vapors.
In addition to image intensifier input phosphors, caesium iodide is often also used in medicine as the scintillating material in flat panel x-ray detectors.
References
- ^ Cesium iodide. U.S. National Library of Medicine
- ^ Haynes, p. 4.57
- Haynes, p. 4.132
- Haynes, p. 10.240
- Huang, Tzuen-Luh; Ruoff, Arthur L. (1984). "Equation of state and high-pressure phase transition of CsI". Physical Review B. 29 (2): 1112. Bibcode:1984PhRvB..29.1112H. doi:10.1103/PhysRevB.29.1112.
- ^ Haynes, p. 5.10
- Kowalski, M. P.; Fritz, G. G.; Cruddace, R. G.; Unzicker, A. E.; Swanson, N. (1986). "Quantum efficiency of cesium iodide photocathodes at soft x-ray and extreme ultraviolet wavelengths". Applied Optics. 25 (14): 2440. Bibcode:1986ApOpt..25.2440K. doi:10.1364/AO.25.002440. PMID 18231513.
- ^ Senga, Ryosuke; Komsa, Hannu-Pekka; Liu, Zheng; Hirose-Takai, Kaori; Krasheninnikov, Arkady V.; Suenaga, Kazu (2014). "Atomic structure and dynamic behaviour of truly one-dimensional ionic chains inside carbon nanotubes". Nature Materials. 13 (11): 1050–4. Bibcode:2014NatMa..13.1050S. doi:10.1038/nmat4069. PMID 25218060.
- Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. Bibcode:1951AcCry...4..487S. doi:10.1107/S0365110X51001641.
- Haynes, p. 5.191
- Mikhailik, V.; Kapustyanyk, V.; Tsybulskyi, V.; Rudyk, V.; Kraus, H. (2015). "Luminescence and scintillation properties of CsI: A potential cryogenic scintillator". Physica Status Solidi B. 252 (4): 804–810. arXiv:1411.6246. Bibcode:2015PSSBR.252..804M. doi:10.1002/pssb.201451464. S2CID 118668972.
- Sun, Da-Wen (2009). Infrared Spectroscopy for Food Quality Analysis and Control. Academic Press. pp. 158–. ISBN 978-0-08-092087-0.
- Lança, Luís; Silva, Augusto (2012). "Digital Radiography Detectors: A Technical Overview" (PDF). Digital Imaging Systems for Plain Radiography. Springer. doi:10.1007/978-1-4614-5067-2_2. hdl:10400.21/1932. ISBN 978-1-4614-5066-5. Archived from the original (PDF) on 2019-01-28. Retrieved 2017-08-28.
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. ISBN 1-4398-5511-0.
| Caesium compounds | |
|---|---|
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||