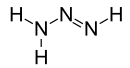| |||
| Names | |||
|---|---|---|---|
| IUPAC name Triazene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| Gmelin Reference | 49028 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | H3N3 | ||
| Molar mass | 45.045 g·mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Other anions | Triphosphane | ||
| Related Binary azanes | ammonia diazane triazane | ||
| Related compounds | Diazene Tetrazene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Triazene is an unsaturated inorganic compound having the chemical formula N3H3. It has one double bond and is the second-simplest member of the azene class of hydronitrogen compounds, after diimide. Triazenes are a class of organic compounds containing the functional group −N(H)−N=N−. Triazene, possibly along with its isomer triimide (HNNHNH), has been synthesized in electron-irradiated ices of ammonia and ammonia/dinitrogen and detected in the gas phase after sublimation.
References
- M. Förstel; Y. A. Tsegaw; P. Maksyutenko; A. M. Mebel; W. Sander; R. I. Kaiser (2016). "On the formation of N3H3 isomers in irradiated ammonia bearing ices: Triazene (H2NNNH) or Triimide (HNHNNH)". ChemPhysChem. 17 (17): 2726–2735. doi:10.1002/cphc.201600414.
External links
| Binary compounds of hydrogen | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali metal (Group 1) hydrides | |||||||||||||||||||
| Alkaline (Group 2) earth hydrides |
| ||||||||||||||||||
| Group 13 hydrides |
| ||||||||||||||||||
| Group 14 hydrides |
| ||||||||||||||||||
| Pnictogen (Group 15) hydrides |
| ||||||||||||||||||
| Hydrogen chalcogenides (Group 16 hydrides) |
| ||||||||||||||||||
| Hydrogen halides (Group 17 hydrides) |
| ||||||||||||||||||
| Transition metal hydrides | |||||||||||||||||||
| Lanthanide hydrides | |||||||||||||||||||
| Actinide hydrides | |||||||||||||||||||
| Exotic matter hydrides | |||||||||||||||||||